|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Stefano ANGIARI
|
| Acad. Degree:
| Ass.-Prof. PhD
|
| Current Position:
| Tenure Track Assistant Professor
Otto Loewi Research Center
Division of Immunology (2nd Deputy Chair)
Medical University of Graz, Austria
|
| Contact Details:
|
Otto Loewi Research Centre, Division of Immunology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
phone: +43 316 385 71172,
✉ e-mail
|
| Websites:
|
[Otto Loewi Research Centre]
[Immunology]
[Team]
[Personal]
|
| ORCID:
|
[0000-0003-4434-2224]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
During the past 19 years of research activity, I have gained extensive expertise in T-cell biology, leukocyte trafficking,
neuroinflammation, autoimmunity and immunometabolism. My research initially focused on the investigation of the molecular mechanisms
controlling lymphocyte activation in secondary lymphoid organs and leukocyte trafficking in homeostatic and inflammatory conditions.
My work led or contributed to: (i) the discovery of the role of mucin P-selectin glycoprotein ligand-1
(PSGL-1) in the functionality of regulatory T cells [1]; (ii) the identification of T-cell
immunoglobulin- and mucin-domain-containing molecule 1 (TIM-1) as a novel trafficking receptor expressed by
pro-inflammatory T helper 1 (Th1) and Th17 cells and mediating their recruitment to the inflamed tissue [2];
(iii) the discovery of novel pathogenic mechanisms in epilepsy, Alzheimer’s disease and cystic fibrosis
[3, 4, 5].
In parallel, I have also been involved in several studies aimed at evaluating the immunomodulatory and neuroprotective capacity of
adipose-derived mesenchymal stem cells (ASCs) in neurological diseases. In these projects, I directly contributed to highlighting the
therapeutic potential of ASCs and ASC-secreted exosomes in neuroinflammation and neurodegeneration [e. g.
6, 7].
In recent years, I became interested in the field of immunometabolism, in particular in how metabolic pathways may control the generation
and strength of an immune response. My research in the field led to seminal discoveries, including: (i) identification of Pyruvate
Kinase M2 (PKM2) as a crucial player in the activation and pathogenicity of pro-inflammatory Th1 and Th17 cells [8];
(ii) identification of post-translational modifications modulating the functionality of the NLR family pyrin domain containing 3
(NLRP3) inflammasome and Janus kinase 1 (JAK1) [9, 10, 11].
I recently started my independent laboratory at the Medical University of Graz (Med Uni Graz), as Tenure Track Assistant Professor. In
my team, we investigate several aspects of cellular and systemic immunometabolism, with a particular focus on: (i) the regulation of
immune-cell function by extracellular metabolites; (ii) the role of metabolic enzyme moonlighting in immune cell activation and
pathogenic potential; (iii) the metabolic profile of immune cells in autoimmune inflammation. We are currently developing several
projects on these topics, in collaboration with both pre-clinical and clinical researchers at Med Uni Graz, as well as with international
research partners (see below).
|
Proposed Dissertation Topic:
The CoA synthesis pathway as a master regulator of immune cell inflammatory potential
Background:
Intracellular metabolic remodelling governs the outcome of immune responses and contributes to the development of inflammation by controlling
leukocyte functionality [12]. Targeting immune-cell metabolism is indeed emerging as a novel and intriguing approach to
boost or limit leukocyte pro-inflammatory potential in several pathogenic contexts [13, 14].
Preliminary results from our laboratory suggest that the coenzyme A (CoA) synthesis pathway is altered in antigen-specific encephalitogenic
T cells inducing experimental autoimmune encephalomyelitis (EAE), the mouse model of multiple sclerosis (MS). Intriguingly, CoA fuelling
limits autoreactive T-cell pathogenicity in vitro and in vivo, as well as the pro-inflammatory profile of T cells
from MS patients (manuscript submitted). These data highlight the potential of such an approach for the treatment of autoimmune neuroinflammation.
Hypothesis and objectives:
Targeting the CoA synthesis pathway in T lymphocytes has recently emerged as a valuable strategy for immunomodulation [15].
However, previous studies reported contrasting results on the effect of CoA fuelling in T cells, depending on the disease context
[16, 17, 18]. Moreover, how this pathway is regulated in other
immune cells during inflammatory responses is unknown. In this project, we will investigate in detail the modulation of the CoA synthesis pathway
in different murine and human immune cells and in vivo experimental settings. In parallel, we will also evaluate whether such a pathway
is dysregulated in leukocytes from patients with neoplastic, autoimmune, and inflammatory diseases.
Methods and approaches:
The PhD candidate will first characterise the time-, activation-, and polarisation-dependent expression of the enzymes catalysing CoA synthesis
from vitamin B5 in different adaptive and innate murine and human leukocyte subsets in vitro (e. g.,
T helper cells, macrophages, dendritic cells). By using qPCR and western blot, they will evaluate these parameters in resting conditions and
at different time points of activation. In parallel, using mass spectrometry, they will assess the flux through the CoA synthesis pathway in resting
and activated immune cells (1st and 2nd year). Based on such results, they will explore the effect of CoA fuelling with CoA precursors
(e. g., vitamin B5, pantethine) or knock out / overexpression of enzymes controlling CoA synthesis
on the pro- or anti-inflammatory activity of adaptive and innate immune cell subsets in vitro. To this purpose, a range of functional
assays will be employed, including qPCR, CRISPR-Cas9, mRNA sequencing, flow cytometry, and multiplex cytokine quantification (1st to 3rd year).
They will then test such pharmacological and / or genetic approaches in mouse models of inflammation, including EAE, allergic asthma,
cancer, and skin inflammation (2nd to 4th year). Finally, using functional assays and mass spectrometry, they will analyse the expression of
CoA-producing enzymes and the rates of CoA synthesis in circulating leukocytes from patients with inflammatory and autoimmune disorders
(e. g. MS, rheumatoid arthritis, asthma / allergy, psoriasis), as well as in immune cells isolated from
tumour masses of cancer patients (2st to 4rd year).
Pitfalls and alternative approaches:
From an experimental point of view, all the techniques to be employed in the study, including animal models, are routinely performed in our
laboratory and by our collaborators. We are actively collaborating with the mass spectrometry facility at Med Uni Graz, where untargeted mass
spectrometry on immune cells is already established. In case we will not be able to detect all the intermediates of the CoA synthesis pathway by
untargeted mass spectrometry, we will move to a more specific and sensitive targeted approach. In any case, the untargeted approach will give us
a broader overview on the metabolic changes taking place in the several immune cells subsets, which may provide backup projects for the student.
Regarding human samples, for most of the pathologies we will investigate peripheral blood mononuclear cells (PBMCs) that have already been collected
and stored for other studies. In case we will need more donors, we will apply for a specific ethical approval for the current study, to recruit
more patients.
Involved Faculty members:
Stefano Angiari (PI), Julia Kargl (tumour mouse models; cancer patient samples, tumour-infiltrating immune cells), Eva Sturm (mouse models
of lung inflammation; blood samples from allergic patients), Peter Wolf (mouse models of skin inflammation; leukocytes from psoriasis patients),
Michael Khalil and Martin Stradner (samples of autoimmune diseases patients).
International Collaborations:
Luke O'Neill (Trinity College Dublin, IE), Dirk Brenner (University of Luxembourg, LU), Gabriela Constantin (University of Verona, IT),
Eleonora Vannini (National Research Council, Pisa, IT).
Facilities:
Our team currently consists of one research technician, one postdoc, one PhD candidate, and three master's students. Our unit is located
at the Division of Immunology of the Otto Loewi Research Center, and is equipped with all the necessary tools for the development of the
project, such as laboratory instrumentation for classic biochemistry, molecular biology and cellular biology studies, including western blot
equipment, thermocyclers for conventional PCR and qPCR, incubators, cell culture hoods, centrifuges, plate readers for fluorometric and
colorimetric assays, and a BD LSRFortessa flow cytometer (BD Biosciences). Med Uni Graz is a high-quality university medical centre, with,
among others, imaging (including several flow cytometers and microscopes), molecular biology (including single cell sequencing equipment),
and mass-spectrometry facilities (link). We will
have full access to all these facilities, in collaboration (free use) or after the payment of use-based fees.
Preparatory Findings:
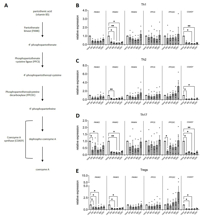 Click to enlarge in new window
Click to enlarge in new window
Figure 1: Modulation of the CoA synthesis pathway in activated human T cells.
(A) The CoA synthesis pathway. The intermediates and the enzymes catalyzing every step are shown. COASY catalyzes the last two steps
of the pathway. (B–E) Naïve CD4+ T cells were activated in vitro in the presence of polarizing
cytokines to generate T helper 1 (Th1) (B), Th2 (C), Th17 (D), and induced regulatory T cells
(Tregs) (E). The expression of all the enzymes involved in the pathway was evaluated by qPCR. For PANK, we assessed the expression of
the 4 known mammalian isoforms (PANK1–4; PANK1 is not shown as it is barely detectable in T cells). Our preliminary data show that
in all the subsets analyzed PANK3 and COASY are strongly downregulated upon activation, while other enzymes show some level of subset-specificity
(e. g., PPCDC downregulation in Th17 cells). Data are the mean ± standard error of the mean (SEM) of
n = 4. *p < 0.05 and **p < 0.01 by
Friedman test.
|
|
References:
-
Angiari S*, Rossi B*, Piccio L, Zinselmeyer BH, Budui S, Zenaro E, Della Bianca V, Bach SD, Scarpini E, Bolomini-Vittori M,
Piacentino G, Dusi S, Laudanna C, Cross AH, Miller MJ, Constantin G:
Regulatory T Cells Suppress the Late Phase of the Immune Response in Lymph Nodes through P-Selectin Glycoprotein Ligand-1.
J Immunol,
2013; 191(11):5489–5500. (*equal contribution)
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Angiari S, Donnarumma T, Rossi B, Dusi S, Pietronigro E, Zenaro E, Della Bianca V, Toffali L, Piacentino G, Budui S, Rennert P, Xiao S, Laudanna C, Casasnovas JM, Kuchroo VK, Constantin G:
TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates effector T cell trafficking during inflammation and autoimmunity.
Immunity,
2014; 40(4):542–553.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G:
A role for leukocyte-endothelial adhesion mechanisms in epilepsy.
Nat Med,
2008; 14(12):1377–1383.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nanì S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G:
Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via a mechanism dependent on LFA-1 integrin.
Nat Med,
2015; 21(8):880–886.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Sorio C, Montresor A, Bolomini-Vittori M, Caldrer S, Rossi B, Dusi S, Angiari S, Johansson JE, Vezzalini M, Leal T, Calcaterra E,
Assael BM, Melotti P, Laudanna C:
Mutations of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene Cause a Monocyte-selective Adhesion Deficiency.
Am J Respir Crit Care Med,
2016; 193(10):1123–1133.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galiè M,
Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B:
Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis.
Stem Cells,
2009; 27(10):2624–2635.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Farinazzo A*, Angiari S*, Turano E*, Bistaffa E, Dusi S, Ruggieri S, Bonafede R, Mariotti R, Constantin G, Bonetti B:
Nanovesicles from adipose-derived mesenchymal stem cells inhibit T lymphocyte adhesion and ameliorate chronic experimental autoimmune encephalomyelitis.
Sci Rep,
2018; 8(1):7473. (*equal contribution)
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Angiari S, Runtsch MC, Sutton CE, Palsson-McDermott EM, Kelly B, Rana N, Kane H, Papadopoulou G, Pearce EL, Mills KHG, O'Neill LAJ:
Pharmacological Activation of Pyruvate Kinase M2 Inhibits CD4+ T Cell Pathogenicity and Suppresses Autoimmunity.
Cell Metab,
2020; 31(2):391–405.e8.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Hughes MM*, Hooftman A*, Angiari S, Tummala P, Zaslona Z, Runtsch MC, McGettrick AF, Sutton CE, Diskin C, Rooke M, Takahashi S,
Sundararaj S, Casarotto MG, Dahlstrom JE, Palsson-McDermott EM, Corr SC, Mills KHG, Preston RJS, Neamati N, Xie Y, Baell JB,
Board PG, O'Neill LAJ:
Glutathione Transferase Omega-1 Regulates NLRP3 Inflammasome Activation through NEK7 Deglutathionylation.
Cell Rep,
2019; 29(1):151–161.e5. (*equal contribution)
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, Ruzek MC, Slivka PF, McGettrick AF, Banahan K, Hughes MM,
Irvine AD, Fischer R, O'Neill LAJ:
The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation.
Cell Metab,
2020; 32(3):468–478.e7.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Runtsch MC, Angiari S, Hooftman A, Wadhwa R, Zhang Y, Zheng Y, Spina JS, Ruzek MC, Argiriadi MA, McGettrick AF, Mendez RS, Zotta A,
Peace CG, Walsh A, Chirillo R, Hams E, Fallon PG, Jayamaran R, Dua K, Brown AC, Kim RY, Horvat JC, Hansbro PM, Wang C,
O'Neill LAJ:
Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages.
Cell Metab,
2022; 34(3):487–501.e8.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Makowski L, Chaib M, Rathmell JC:
Immunometabolism: From basic mechanisms to translation.
Immun Rev,
2020; 295(1):5–14.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Patel CH, Leone RD, Horton MR, Powell JD:
Targeting metabolism to regulate immune responses in autoimmunity and cancer.
Nat Rev Drug Discov,
2019; 18(9):669–688.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Bader JE, Voss K, Rathmell JC:
Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy.
Mol Cell,
2020; 78(6):1019–1033.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Miallot R, Millet V, Galland F, Naquet P:
The vitamin B5/coenzyme A axis: A target for immunomodulation?
Eur J Immunol,
2023; 53(10):e2350435.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Abou-Hamdan M, Gharib B, Bajenoff M, Julia V, de Reggi M:
Pantethine Down-Regulates Leukocyte Recruitment and Inflammatory Parameters in a Mouse Model of Allergic Airway Inflammation.
Med Sci Monit Basic Res,
2017; 23:368–372.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Miallot R, Millet V, Roger A, Fenouil R, Tardivel C, Martin JC, Shintu L, Berchard P, Sousa Lanza J, Malissen B, Henri S, Ugolini S, Dutour A,
Finetti P, Bertucci F, Blay JY, Galland F, Naquet P:
The coenzyme A precursor pantethine enhances antitumor immunity in sarcoma.
Life Sci Alliance,
2023; 6(12):e202302200.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
St Paul M, Saibil SD, Han S, Israni-Winger K, Lien SC, Laister RC, Sayad A, Penny S, Amaria RN, Haydu LE, Garcia-Batres CR, Kates M,
Mulder DT, Robert-Tissot C, Gold MJ, Tran CW, Elford AR, Nguyen LT, Pugh TJ, Pinto DM, Wargo JA, Ohashi PS:
Coenzyme A fuels T cell anti-tumor immunity.
Cell Metab,
2021; 33(12):2415–2427.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
|
|





![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)