|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Michael KHALIL
|
| Acad. Degree:
| Assoz. Prof. Priv.-Doz. Dr. med. univ. (MD) PhD
|
| Current Position:
| Specialized physician in Neurology,
Head, Neurology Biomarker Research Unit,
Department of Neurology,
Medical University of Graz
|
| Contact Details:
|
phone: +43 316 385 30313,
✉ e-mail
| Websites:
|
[Department]
[Team]
[Personal]
|
| ORCID:
|
[0000-0002-5350-3328]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
The Neurology Biomarker Research Unit was founded at the Department of Neurology by Michael Khalil in 2015. Michael Khalil is a clinical
and translational neuroscientist with a focus on body fluid and imaging biomarkers in neuroimmunological and neurodegenerative disorders.
He has a long-standing experience in cerebrospinal fluid diagnostics and clinical neurochemistry with a strong international research
network facilitating to perform large multi-centre biomarker discovery and validation studies. The research focuses on pathophysiological
mechanisms of neuroimmunological disorders, with particular interest in multiple sclerosis (MS). The main aim of the Research Unit is to
develop and validate body fluid biomarkers for disease progression, different disease phases/stages and response to disease-modifying
treatments. By this means our group investigates biochemical factors in the cerebrospinal fluid (CSF), blood (including DNA, RNA, and
peripheral blood mononuclear cells, PBMCs) and urine in patients with neurological disorders.
Research findings are being evaluated and interpreted in strong association with imaging (conventional and non-conventional MRI at 3T)
findings of the central nervous system and detailed clinical follow-up data. This combined approach shall ultimately serve to define
patient subgroups and aid in the development of more effective treatment regimens and drugs.
Current research focuses on investigating the clinical significance of cerebrospinal fluid and blood based biomarkers using ultra-high
sensitive detection techniques in neuroimmunological and neurodegenerative disorders.
|
Proposed Dissertation Topic:
Immunologic and metabolic profiles related to progression independent of relapse activity (PIRA) in patients with multiple sclerosis
Background:
In the chronic immune mediated disease multiple sclerosis (MS), irreversible accumulation of disability can occur at any stage of the
disease and may be driven by two main mechanisms, that are relapse-associated worsening (RAW) and progression independent of relapse
activity (PIRA). Especially PIRA, which has recently been described as a common feature in MS, seems to be the main driving factor for
disease progression in MS without formal diagnosis of a secondary progressive disease course. It is now clear that PIRA can occur even in
very early stages of the disease, e. g. in patients after a first demyelinating attack of the central nervous system
but also frequently in established MS. Recently, the clinical and neuroimaging predictors of PIRA at the time of the first demyelinating
event have been investigated. Patients with PIRA had more brain lesions and had a steeper yearly increase in physical disability. However,
the immunological mechanisms underlying PIRA are still unclear.
Hypothesis and objectives:
We hypothesize that different phenotypic and metabolic profiles in circulating immune cells may be related to PIRA and may allow to
identify patients at risk for early disease worsening. Early identification of immunological mechanisms underlying disease progression is
of great importance for treatment decisions to ensure better long-term outcomes and discover novel therapeutic targets. The PhD candidate
will investigate whether (i) the inflammatory profile and metabolic features of circulating immune cells allow to identify patients
with PIRA; (ii) the inflammatory profile and metabolic features of circulating immune cells correlate with soluble markers of
inflammation and neurodegeneration in cerebrospinal fluid and blood; (iii) immunological and clinical characteristics explain
MS-related signs of tissue damage as evidenced by magnetic resonance imaging (MRI).
Methods and approaches:
The PhD candidate will be trained in isolation of immune cell subsets from human blood (1st year). To potentially link disease
worsening to immunological events in MS, the student will perform comprehensive immunophenotyping of the immune cells by flow
cytometry, looking at (among others): (i) activation markers (e. g., CD71, CD25, CD69); (ii) cytokine
production (e. g., GM-CSF, IL-17A, IFN-γ); (iii) markers of cell
senescence (e. g., CD28) (1st to 3rd year). The student will also determine the overall metabolic profile of
T cells, B cells, and monocytes by Seahorse assay, mass spectrometry, immunofluorescence, flow cytometry, qPCR, and
western blot (2nd and 3rd year). Examples of readouts are mitochondrial mass and activity, glucose uptake and glycolytic capacity,
expression of metabolite transporters and receptors (e. g. GLUT1, SUCNR1, MCT-4), expression
and / or activity of metabolic enzymes and sensors (e. g., LDHA, PKM, MTOR, CPT1),
accumulation and secretion of metabolic intermediates (e. g. succinate, lactate, fumarate). The immunologic,
metabolic and cellular profiles will then be correlated with clinical and MRI data and soluble markers of inflammation and
neurodegeneration in the cerebrospinal fluid and blood (3rd and 4th year).
Pitfalls and alternative approaches:
All laboratory tests included in this study have already been established in the participating institutions. Samples will be processed
according to international consensus protocols to ensure pre-analytical stability. Comprehensive clinical and MRI data, including post-processing,
will be collected according to established protocols. Data documentation will be performed using the database already established at our
university. Ethical approval has been obtained to conduct this study. Regular team meetings will be scheduled to ensure early and continuous
risk assessment and implementation of effective mitigation strategies.
Involved Faculty members:
Michael Khalil (PI), Stefano Angiari (immune cell phenotyping, metabolic profile),
Martin Stradner (functional immune cell assays), Vanessa
Stadlbauer-Kööllner (neutrophil assays), and Julia Kargl and Eva Sturm
(flow cytometry).
International Collaborations:
Jens Kuhle (Basel, CH), Charlotte Teunissen (Amsterdam, NL), Manuel Comabella (Barcelona, ES).
Facilities:
Our team currently consists of two medical doctors, three PhD candidates and one technicians. Our laboratory is located at 2nd floor
of the Department of Neurology at the Medical University of Graz. Our laboratory is equipped with up to date fully automated ultra-high
sensitive analytical platforms, such as the Simoa HD-X AnalyzerTM from Quanterix. Neurodegenerative markers in the CSF are further
being analyzed by the automated Lumipulse® G600II from Fujirebio. CSF and Serum protein analysis are performed on the
Optilite® analyzer from The Binding Site. Our department provides the required laboratory and office space, basic
laboratory facilities, such as immunofluorescence microscopy, isolating fresh human peripheral blood leukocytes from blood donors,
DNA extraction and more. Flow cytometry analysis are mainly performed at the Division of Immunology and the Division of Rheumatology and
Immunology. Seahorse technology, metabolomics and mass spectrometry are also available on the campus through collaboration partners.
Preparatory Findings:
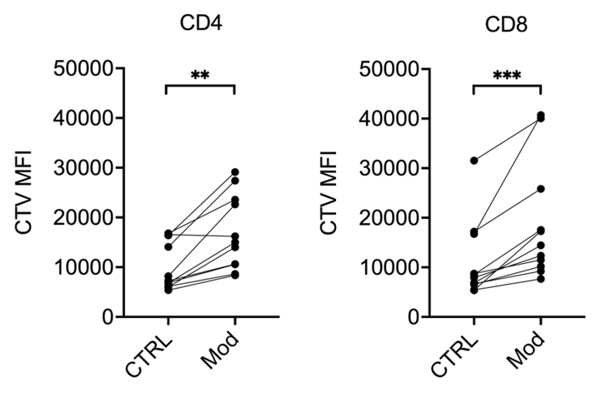
Figure 1. A metabolic modulator limits proliferation of T cells from multiple sclerosis (MS) patients.
Peripheral blood mononuclear cells (PBMCs) from MS patients were stained with fluorescent Cell-Trace Violet (CTV) and restimulated
in vitro with anti-CD3 / anti-CD28 antibodies, in the presence of vehicle (CTRL) or a metabolic modulator
(Mod; undisclosed). After 5 days of culture, cells were stained with anti-CD3, anti-CD4, and anti-CD8 antibodies and analyzed by
flow cytometry. As the cells divide, CTV is diluted in daughter cells. Thus, the lowest the CTV fluorescence intensity, the higher the
cells proliferation. Mod significantly increased CTV mean fluorescence intensity (MFI) in both CD4+ and CD8+
T cells, suggesting less proliferative capacity of cells treated with Mod. ** p < 0.01
and *** p < 0.001 by Wilcoxon signed-rank test; n = 13.
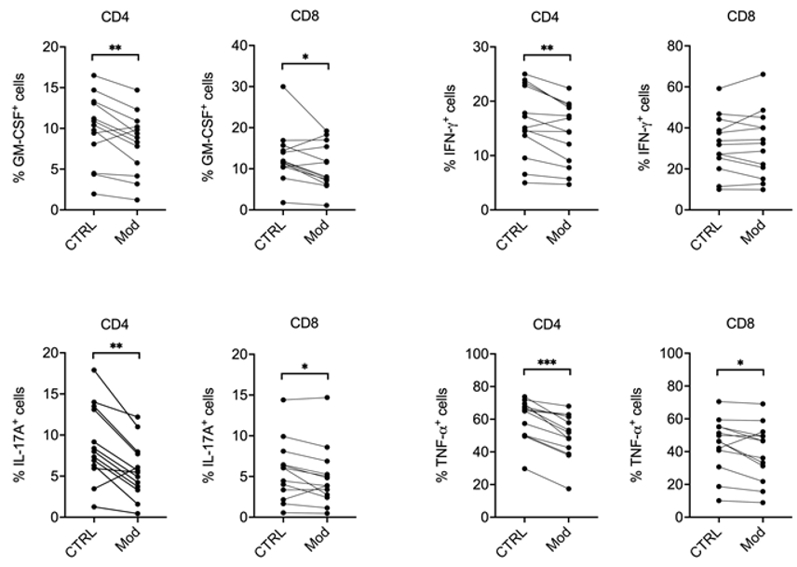
Figure 2. A metabolic modulator limits pro-inflammatory cytokine production by MS T cells.
PBMCs from MS patients were restimulated in vitro with anti-CD3 / anti-CD28 antibodies,
in the presence of vehicle (CTRL) or a metabolic modulator (Mod; undisclosed). After 24 hours of culture, cells were restimulated
with PMA / ionomycin / brefeldin A for 5%bvsp;hours. Cells were then collected and stained with
anti-CD3, anti-CD4, and anti-CD8 antibodies plus antibodies against several pro-inflammatory
cytokines relevant for MS pathogenesis, and analyzed by flow cytometry. Our results indicate that Mod reduces the percentage of
CD4+ and CD8+ T cells producing granulocyte-macrophage colony-stimulating factor (GM-CSF),
interferon-gamma (IFN-γ), interleukin-17A (IL-17A), and tumour necrosis factor-alpha
(TNF-α). * p < 0.05, ** p < 0.01, and
*** p < 0.001 by Wilcoxon signed-rank test; n = 13.
|
|
References:
-
Buchmann A, Pirpamer L, Pinter D, Voortman M, Helmlinger B, Pichler A, Maceski AM, Benkert P, Bachmaier G, Ropele S, Reindl M,
Leppert D, Kuhle J, Enzinger C, Khalil M:
High serum neurofilament light chain levels correlate with brain atrophy and physical disability in multiple sclerosis.
Eur J Neurol,
2023; 30(5):1389–1139.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Meier S, Willemse EAJ, Schaedelin S, Oechtering J, Lorscheider J, Melie-Garcia L, Cagol A, Barakovic M, Galbusera R, Subramaniam S,
Barro C, Abdelhak A, Thebault S, Achtnichts L, Lalive P, Müller S, Pot C, Salmen A, Disanto G, Zecca C, D’Souza M, Orleth A,
Khalil M, Buchmann A, Du Pasquier R, Yaldizli Ö, Derfuss T, Berger K, Hermesdorf M, Wiendl H, Piehl F, Battaglini M,
Fischer U, Kappos L, Gobbi C, Granziera C, Bridel C, Leppert D, Maleska Maceski A, Benkert P, Kuhle J:
Serum Glial Fibrillary Acidic Protein Compared With Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple
Sclerosis.
JAMA Neurol,
2023; 80(3):287–297.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Cagol A, Schaedelin S, Barakovic M, Benkert P, Todea RA, Rahmanzadeh R, Galbusera R, Lu PJ, Weigel M, Melie-Garcia L, Ruberte E,
Siebenborn N, Battaglini M, Radue EW, Yaldizli Ö, Oechtering J, Sinnecker T, Lorscheider J, Fischer-Barnicol B, Müller S,
Achtnichts L, Vehoff J, Disanto G, Findling O, Chan A, Salmen A, Pot C, Bridel C, Zecca C, Derfuss T, Lieb JM, Remonda L, Wagner F,
Vargas MI, Du Pasquier R, Lalive PH, Pravatà E, Weber J, Cattin PC, Gobbi C, Leppert D, Kappos L, Kuhle J, Granziera C:
Association of Brain Atrophy With Disease Progression Independent of Relapse Activity in Patients With Relapsing Multiple Sclerosis.
JAMA Neurol,
2022; 79(7):682–692.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Weiss B, Pichler A, Damulina A, Buchmann A, Hochmeister S, Seifert-Held T, Enzinger C, Archelos JJ, Khalil M:
Evidence for an Intrathecal Immunoglobulin Synthesis by Kappa Free Light Chains in Neurological Patients with an Isolated Band in
Isoelectric Focusing.
Biomedicines,
2022; 10(9):2202.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Voortman MM, Damulina A, Pirpamer L, Pinter D, Pichler A, Enzinger C, Ropele S, Bachmaier G, Archelos JJ, Marsche G, Khalil M:
Decreased Cerebrospinal Fluid Antioxidative Capacity Is Related to Disease Severity and Progression in Early Multiple Sclerosis.
Biomolecules,
2021; 11(9):1264.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Koini M, Pirpamer L, Hofer E, Buchmann A, Pinter D, Ropele S, Enzinger C, Benkert P, Leppert D, Kuhle J, Schmidt R, Khalil M:
Factors influencing serum neurofilament light chain levels in normal aging.
Aging (Albany NY),
2021; 13(24):25729–25738.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, Model F, Koendgen H, Manfrini M, Belachew S, Hauser SL:
Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in
Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials.
JAMA Neurol,
2020; 77(9):1132–1140.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, Benkert P, Ropele S, Enzinger C, Fazekas F, Schmidt R, Kuhle J:
Serum neurofilament light levels in normal aging and their association with morphologic brain changes.
Nat Commun,
2020; 11(1):812.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Angiari S, Runtsch MC, Sutton CE, Palsson-McDermott EM, Kelly B, Rana N, Kane H, Papadopoulou G, Pearce EL, Mills KHG,
O'Neill LAJ:
Pharmacological Activation of Pyruvate Kinase M2 Inhibits CD4+ T Cell Pathogenicity and Suppresses Autoimmunity.
Cell Metab,
2020; 31(2):391–405.e8.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Pinter D, Gattringer T, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, Eppinger S, Pirpamer L, Bachmaier G,
Ropele S, Wardlaw JM, Kuhle J, Khalil M, Fazekas F:
Longitudinal MRI dynamics of recent small subcortical infarcts and possible predictors.
J Cereb Blood Flow Metab,
2019; 39(9):1669–1677.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE; and the NFL Group; Alvarez-Cermeño JC, Andreasson U,
Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialová L,
Forsgren L, Frederiksen JL, Gisslén M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J,
Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landén M, Leinonen V, Logroscino G, Lu CH,
Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Pérez-Santiago J,
Piehl F, Pijnenburg YAL, Pyykkö OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM,
Sellebjerg FT, Simone IL, Skillbäck T, Stilund M, Sundström P, Svenningsson A, Tortelli R, Tortorella C, Trentini A,
Troiano M, Turner MR, van Swieten JC, Vågberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelsø C,
Wild EJ:
Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis.
JAMA Neurol,
2019; 76(9):1035–1048.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K,
Zetterberg H, Kuhle J:
Neurofilaments as biomarkers in neurological disorders.
Nat Rev Neurol,
2018; 14(10):577–589.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Khalil M, Renner A, Langkammer C, Enzinger C, Ropele S, Stojakovic T, Scharnagl H, Bachmaier G, Pichler A, Archelos JJ, Fuchs S,
Seifert-Held T, Fazekas F:
Cerebrospinal fluid lipocalin 2 in patients with clinically isolated syndromes and early multiple sclerosis.
Mult Scler,
2016; 22(12):1560–1568.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Khalil M, Langkammer C, Pichler A, Pinter D, Gattringer T, Bachmaier G, Ropele S, Fuchs S, Enzinger C, Fazekas F:
Dynamics of brain iron levels in multiple sclerosis: A longitudinal 3T MRI study.
Neurology,
2015; 84(24):2396 2402.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
O'Neill LA, Kishton RJ, Rathmell J:
A guide to immunometabolism for immunologists.
Nat Rev Immunol,
2016; 16(9):553–565.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Pearce EL, Pearce EJ:
Metabolic pathways in immune cell activation and quiescence.
Immunity,
2013; 38(4):633–643.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
| |
|






![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)