|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Dagmar KRATKY
|
| Acad. Degree:
| Univ.-Prof. Dr. rer. nat. (PhD)
|
| Current Position:
| Vice chair Molecular Biology and Biochemistry,
Gottfried Schatz Research Center,
Medical University of Graz
|
| Contact Details:
|
Gottfried Schatz Research Centre, Division of Molecular Biology and Biochemistry, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
phone: +43 316 385 71965,
✉ e-mail
|
| Websites:
|
[Gottfried Schatz Research Centre]
[Team]
[SFB Lipid Hydrolysis]
[Personal]
|
| ORCID:
|
[0000-0003-1357-7573]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
Dagmar Kratky is a biochemist and molecular biologist with expertise in lipid and energy metabolism. Over the past years, her laboratory
has successfully explored the role of neutral and acid lipid hydrolases in tissues and cells utilizing mutant mouse models with loss or
overexpression of these enzymes. The group aims to understand the molecular mechanisms that cause abnormalities in lipid and lipoprotein
metabolism associated with the development of metabolic disorders. In addition, they are interested in cell and tissue autonomous functions
of the respective enzymes. Important contributions include the generation and characterization of (tissue-specific) lysosomal acid lipase
(LAL)-deficient mice [1–6], the discovery of off-target effects of LAL inhibitors
[7], the role of adipose triglyceride lipase (ATGL) in the small intestine [8, 9],
and the consequences of the loss of lipid hydrolases in macrophages [10–17] and
atherosclerosis development in mice [18–23].
|
Proposed Dissertation Topic:
Consequences of intestinal ATGL expression on liver inflammation
Background:
The rising incidence of metabolic disorders and cardiovascular diseases in recent decades has been linked to an
increasing intake of dietary fat. Small intestinal (SI) enterocytes play an essential role in maintaining systemic lipid parameters by
regulating dietary lipid absorption and postprandial lipid secretion in chylomicrons or high-density lipoproteins (HDL). Of note, the SI
accounts for approximately 30 % of the steady-state HDL plasma pool. In addition to immediate secretion in lipoproteins,
excess dietary lipids are transiently stored in cytosolic lipid droplets (LDs) that are mobilized upon demand. Lack of intestinal
hormone-sensitive lipase (HSL) leads to CE accumulation in the small intestine, acceleratesd cholesterol absorption and decreased cholesterol
biosynthesis, indicating that HSL plays an important role in intestinal cholesterol homeostasis [24]. We have also
shown that adipose triglyceride lipase (ATGL), together with its coactivator CGI-58, is involved in the hydrolysis of
intestinal LDs without affecting dietary lipid uptake or chylomicron secretion [9, 25].
Unexpectedly, we observed modulations of intestinal and systemic cholesterol homeostasis in intestine-specific Atgl-deficient mice
[9] and in mice overexpressing ATGL exclusively in the SI (Atgl iTg) [8].
Hypothesis and objectives:
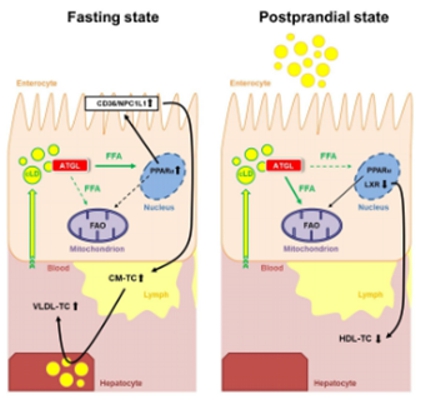 Atgl iTg mice show reduced circulating cholesterol levels in the refed state, mainly
due to reduced HDL cholesterol. Consistent with recent findings that gut-derived HDL plays an important role in protecting against
lipopolysaccharide-, alcohol-, or diet-induced hepatic inflammation [1], Atgl iTg mice exhibit elevated
expression of liver inflammation markers, presumably due to downregulation of genes involved in intestinal HDL secretion. Treatment
with a liver X receptor (LXR) agonist increased HDL secretion in Atgl iTg mice [5] by unknown mechanism(s).
Of note, ATGL is regulated differently in the SI than in other tissues, and the nutritional regulation of intestinal ATGL expression
and its involvement in LXR signaling require further investigation. Since HDL produced by the SI protects the liver from inflammation
[1], compromised signaling in Atgl iTg mice may trigger hepatic inflammation, whereas loss of intestinal ATGL
might be associated with a beneficial inflammatory propensity of the liver. Although our studies have indicated a close link between
intestinal and hepatic lipid metabolism, the interplay between the SI, lipoprotein metabolism, and hepatocytes is incompletely understood.
Atgl iTg mice show reduced circulating cholesterol levels in the refed state, mainly
due to reduced HDL cholesterol. Consistent with recent findings that gut-derived HDL plays an important role in protecting against
lipopolysaccharide-, alcohol-, or diet-induced hepatic inflammation [1], Atgl iTg mice exhibit elevated
expression of liver inflammation markers, presumably due to downregulation of genes involved in intestinal HDL secretion. Treatment
with a liver X receptor (LXR) agonist increased HDL secretion in Atgl iTg mice [5] by unknown mechanism(s).
Of note, ATGL is regulated differently in the SI than in other tissues, and the nutritional regulation of intestinal ATGL expression
and its involvement in LXR signaling require further investigation. Since HDL produced by the SI protects the liver from inflammation
[1], compromised signaling in Atgl iTg mice may trigger hepatic inflammation, whereas loss of intestinal ATGL
might be associated with a beneficial inflammatory propensity of the liver. Although our studies have indicated a close link between
intestinal and hepatic lipid metabolism, the interplay between the SI, lipoprotein metabolism, and hepatocytes is incompletely understood.
Methods and approaches:
The PhD candidate will use enterocyte-specific Atgl/Cgi58-iDKO and Atgl iTg mice to study the
effects of intestinal ATGL on (intestinal) HDL production and liver inflammation. Intestine and liver of Atgl/Cgi58-iDKO, Atgl iTg,
and control mice in various nutritional states fed different diets plus / minus LXR agonists or probiotics will be analyzed
for gene (RNAseq, qPCR) and protein expression (proteomics, western blotting, immunohistochemistry), and microscopy techniques to
identify the underlying mechanism of ATGL-dependent HDL secretion in the SI and its effects on liver inflammation (1st to 3rd year). Flux
analyses using radiolabeled and fluorescently labeled lipids will be used to track their distribution among metabolically active organs
and to elucidate their role in systemic lipid homeostasis (3rd year). Intestinal and liver organoids will be established as a tool
for pharmaceutical interventions (4th year).
Pitfalls and alternative approaches:
Intestinal HDL was shown to be generated most abundantly by the ileum and does not travel into draining lymphatic vessels like enterocyte-derived
chylomicrons do. Instead, intestinal HDL rapidly enters the portal vein, the major blood supply to the liver [26]. Thus,
to investigate whether impaired signaling in Atgl iTg mice triggers hepatic inflammation and whether loss of intestinal ATGL is associated
with reduced hepatic inflammation, plasma must be isolated from the inferior vena cava, which could be a challenge at the beginning.
Involved Faculty members: Dagmar Kratky (PI), Melanie Korbelius (Co-PI); within MET-FLAM:
Julia Kargl (immune cells and flow cytometry), Gunther Marsche (HDL metabolism),
Vanessa Stadlbauer-Köllner (microbiome), Simon Sedej (autophagy assays),
Kathrin Eller (immunological assays).
International Collaborations: Richard Lehner (Edmonton, CA), Erin Kershaw (Pittsburgh, PA), Kimberly Buhman (West Lafayette,
IN), G. Danilo Norata (Milan, Italy), Elizabeth Rendina-Ruedy (Nashville, TN).
Facilities: Dagmar Kratky’s research group has a long history in lipid research. Her team currently consists of two
senior scientists, one postdoc, four PhD students, and five technicians. Her laboratory is located at the newly opened basic research campus
of the university. The local infrastructure includes
cell culture facilities, a radionuclide laboratory for in vitro and in vivo experiments, comprehensive biochemical,
molecular biology, and cell biology facilities, a flow cytometer, real-time PCR instruments, fluorescent and deconvolution microscopes,
the Nikon Center of Excellence for super-resolution microscopy: Cells & Organelles, a Nanolive microscope for label-free imaging of
living cells, gas chromatography-mass spectroscopy, fast performance liquid chromatography, and an XF96 extracellular flux analyzer (Seahorse).
Through many years of collaboration, we have access to nuclear magnetic resonance (NMR) spectroscopy, small-angle X-ray scattering (SAXS),
and mass spectrometry for lipidomics. The animal facility (including on-line metabolic cages for 12 mice plus a temperature-controlled
housing) houses all mouse models relevant for the present proposal. The Center for Medical Research (ZMF, Med Uni Graz) provides
micro-computed tomography, a biological irradiator (RS2000) to perform bone marrow transplantation, an LSRII flow cytometer equipped with
four lasers, and a FACSAria flow cytometer for cell sorting, all of which have already been successfully used by the applicant.
Infrastructure from the University of Graz and the Graz University of Technology is also fully available through the networking initiative
BioTechMed-Graz.
Preparatory Findings:
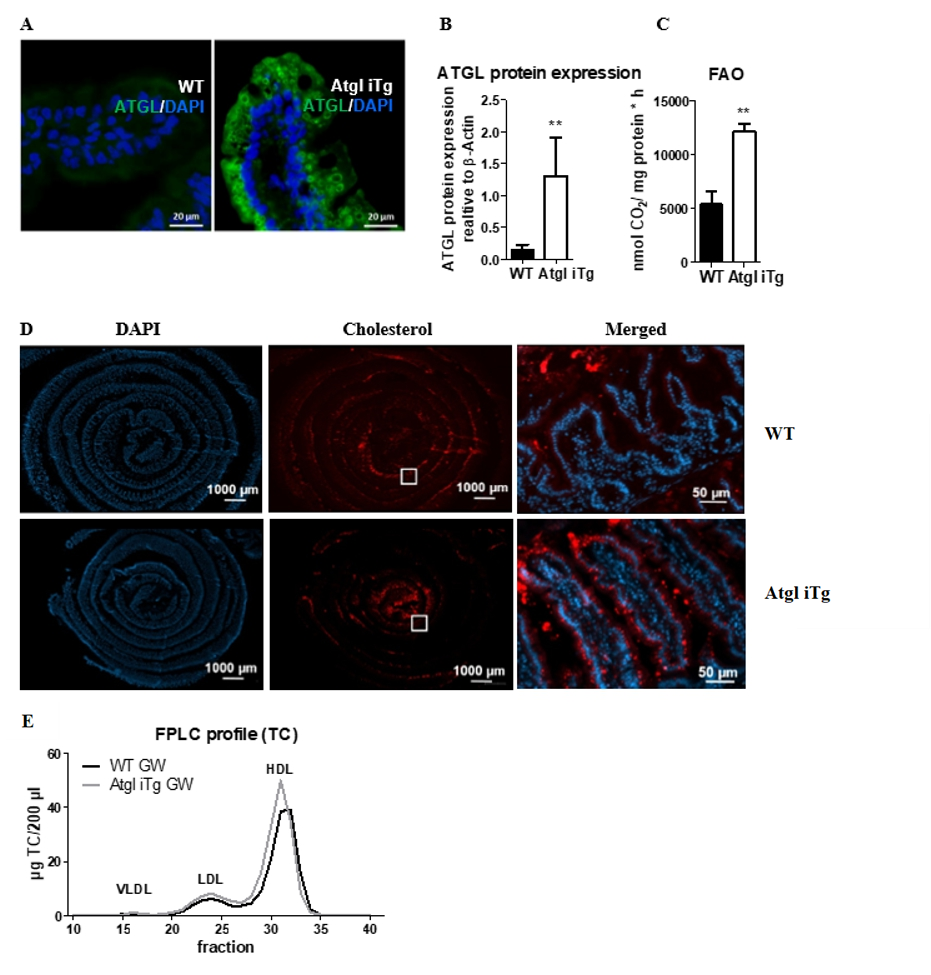
Figure 1. Efficient intestine-specific overexpression of ATGL.
(A) Representative images of ATGL immunofluorescence staining (green) with DAPI (blue) on jejunal cryosections.
Scale bar, 20 µm. (B) Quantification of isolated jejunal enterocytes to detect ATGL protein expression.
(C) Fatty acid oxidation in jejunal enterocytes of chow diet-fed Atgl iTg mice and their corresponding WT littermates.
(D) Cryosections of jejunal swiss rolls (middle to outer layer: proximal to distal part) 2 h after oral administration
of fluorescently-labeled cholesterol (red) with DAPI staining for nuclei (blue) in 14-week-old female mice.
Scale bar, 1000 µm; magnification of square: scale bar, 50 µm.
(E) Total cholesterol lipoprotein profiles after 9 days of treatment with the LXR agonist GW3965. Data represent
mean values (n = 4 6) + SD.
** p < 0.01. Figure adapted from [8].
|
|
References:
-
Sachdev V, Duta-Mare M, Korbelius M, Vujić N, Leopold C, Freark de Boer J, Rainer S, Fickert P, Kolb D, Kuipers F,
Radovic B, Gorkiewicz G, Kratky D:
Impaired Bile Acid Metabolism and Gut Dysbiosis in Mice Lacking Lysosomal Acid Lipase.
Cells,
2021; 10(10): 2619.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Leopold C, Duta-Mare M, Sachdev V, Goeritzer M, Maresch LK, Kolb D, Reicher H, Wagner B, Stojakovic T, Ruelicke T, Haemmerle G,
Hoefler G, Sattler W, Kratky D:
Hepatocyte-specific lysosomal acid lipase deficiency protects mice from diet-induced obesity but promotes hepatic inflammation.
Biochim Biophys Acta Mol Cell Biol Lipids,
2019; 1864(4):500–511.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Viaud M, Ivanov S, Vujic N, Duta-Mare M, Aira LE, Barouillet T, Garcia E, Orange F, Dugail I, Hainault I, Stehlik C, Marchetti S, Boyer L,
Guinamard R, Foufelle F, Bochem A, Hovingh KG, Thorp EB, Gautier EL, Kratky D, Dasilva-Jardine P, Yvan-Charvet L:
Lysosomal Cholesterol Hydrolysis Couples Efferocytosis to Anti-Inflammatory Oxysterol Production.
Circ Res,
2018; 122(10):1369–1384.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Duta-Mare M, Sachdev V, Leopold C, Kolb D, Vujic N, Korbelius M, Hofer DC, Xia W, Huber K, Auer M, Gottschalk B, Magnes C, Graier WF,
Prokesch A, Radovic B, Bogner-Strauss JG, Kratky D:
Lysosomal acid lipase regulates fatty acid channeling in brown adipose tissue to maintain thermogenesis.
Biochim Biophys Acta Mol Cell Biol Lipids,
2018; 1863(4):467–478.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Radović B, Vujić N, Leopold C, Schlager S, Goeritzer M, Patankar JV, Korbelius M, Kolb D, Reindl J, Wegscheider M,
Tomin T, Birner-Gruenberger R, Schittmayer M, Groschner L, Magnes C, Diwoky C, Frank S, Steyrer E, Du H, Graier WF, Madl T,
Kratky D:
Lysosomal acid lipase regulates VLDL synthesis and insulin sensitivity in mice.
Diabetologia,
2016; 59(8):1743–1752.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Bianco V, Korbelius M, Vujic N, Akhmetshina A, Amor M, Kolb D, Pirchheim A, Bradic I, Kuentzel KB, Buerger M, Schauer S, Phan HTT,
Bulfon D, Hoefler G, Zimmermann R, Kratky D:
Impact of (intestinal) LAL deficiency on lipid metabolism and macrophage infiltration.
Mol Metab,
2023; 73:101737.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Bradić I, Kuentzel KB, Honeder S, Grabner GF, Vujić N, Zimmermann R, Birner-Gruenberger R, Kratky D:
Off-target effects of the lysosomal acid lipase inhibitors Lalistat-1 and Lalistat-2 on neutral lipid hydrolases.
Mol Metab,
2022; 61:101510.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Korbelius M, Vujić N, Kuentzel KB, Obrowsky S, Rainer S, Haemmerle G, Rulicke T, Kratky D:
Enterocyte-specific ATGL overexpression affects intestinal and systemic cholesterol homeostasis.
Biochim Biophys Acta Mol Cell Biol Lipids,
2022; 1867(4):159121.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Korbelius M, Vujic N, Sachdev V, Obrowsky S, Rainer S, Gottschalk B, Graier WF, Kratky D:
ATGL/CGI-58-Dependent Hydrolysis of a Lipid Storage Pool in Murine Enterocytes.
Cell Rep,
2019; 28(7):1923–1934.e4.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Schlager S, Vujic N, Korbelius M, Duta-Mare M, Dorow J, Leopold C, Rainer S, Wegscheider M, Reicher H, Ceglarek U, Sattler W,
Radovic B, Kratky D:
Lysosomal lipid hydrolysis provides substrates for lipid mediator synthesis in murine macrophages.
Oncotarget,
2017; 8(25):40037–40051.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Schlager S, Goeritzer M, Jandl K, Frei R, Vujic N, Kolb D, Strohmaier H, Dorow J, Eichmann TO, Rosenberger A, Wolfler A, Lass A,
Kershaw EE, Ceglarek U, Dichlberger A, Heinemann A, Kratky D:
Adipose triglyceride lipase acts on neutrophil lipid droplets to regulate substrate availability for lipid mediator synthesis.
J Leukoc Biol,
2015; 98(5):837–850.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Goeritzer M, Vujic N, Schlager S, Chandak PG, Korbelius M, Gottschalk B, Leopold C, Obrowsky S, Rainer S, Doddapattar P,
Aflaki E, Wegscheider M, Sachdev V, Graier WF, Kolb D, Radovic B, Kratky D:
Active autophagy but not lipophagy in macrophages with defective lipolysis.
Biochim Biophys Acta,
2015; 1851(10):1304–1316.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Aflaki E, Doddapattar P, Radović B, Povoden S, Kolb D, Vujić N, Wegscheider M, Koefeler H, Hornemann T,
Graier WF, Malli R, Madeo F, Kratky D:
C16 ceramide is crucial for triacylglycerol-induced apoptosis in macrophages.
Cell Death Dis,
2012; 3:e280.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Aflaki E, Radović B, Chandak PG, Kolb D, Eisenberg T, Ring J, Fertschai I, Uellen A, Wolinski H, Kohlwein SD, Zechner R,
Levak-Frank S, Sattler W, Graier WF, Malli R, Madeo F, Kratky D:
Triacylglycerol accumulation activates the mitochondrial apoptosis pathway in macrophages.
J Biol Chem,
2011; 286(9):7418–7428.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Aflaki E, Balenga NA, Luschnig-Schratl P, Wolinski H, Povoden S, Chandak PG, Bogner-Strauss JG, Eder S, Konya V, Kohlwein SD,
Heinemann A, Kratky D:
Impaired Rho GTPase activation abrogates cell polarization and migration in macrophages with defective lipolysis.
Cell Mol Life Sci,
2011; 68(23):3933–3947.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Chandak PG, Radović B, Aflaki E, Kolb D, Buchebner M, Frohlich E, Magnes C, Sinner F, Haemmerle G, Zechner R, Tabas I,
Levak-Frank S, Kratky D:
Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase.
J Biol Chem,
2010; 285(26):20192–20201.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Buchebner M, Pfeifer T, Rathke N, Chandak PG, Lass A, Schreiber R, Kratzer A, Zimmermann R, Sattler W, Koefeler H, Fröhlich E,
Kostner GM, Birner-Gruenberger R, Chiang KP, Haemmerle G, Zechner R, Levak-Frank S, Cravatt BF, Kratky D:
Cholesteryl ester hydrolase activity is abolished in HSL−/− macrophages but unchanged in macrophages lacking KIAA1363.
J Lipid Res,
2010; 51:2896–2908.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Vujic N, Schlager S, Eichmann TO, Madreiter-Sokolowski CT, Goeritzer M, Rainer S, Schauer S, Rosenberger A, Woelfler A,
Doddapattar P, Zimmermann R, Hoefler G, Lass A, Graier WF, Radovic B, Kratky D:
Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice.
Atherosclerosis,
2016; 244(1):9–21.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Vujic N, Porter Abate J, Schlager S, David T, Kratky D, Koliwad SK:
Acyl-CoA : Diacylglycerol Acyltransferase 1 Expression Level in the Hematopoietic Compartment Impacts Inflammation in the
Vascular Plaques of Atherosclerotic Mice.
PLoS One,
2016; 11(5):e0156364.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Goeritzer M, Schlager S, Radovic B, Madreiter CT, Rainer S, Thomas G, Lord CC, Sacks J, Brown AL, Vujic N, Obrowsky S,
Sachdev V, Kolb D, Chandak PG, Graier WF, Sattler W, Brown JM, Kratky D:
Deletion of CGI-58 or adipose triglyceride lipase differently affects macrophage function and atherosclerosis.
J Lipid Res,
2014; 55(12):2562–2575.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Lammers B, Chandak PG, Aflaki E, Van Puijvelde GH, Radovic B, Hildebrand RB, Meurs I, Out R, Kuiper J, Van Berkel TJ,
Kolb D, Haemmerle G, Zechner R, Levak-Frank S, Van Eck M, Kratky D:
Macrophage adipose triglyceride lipase deficiency attenuates atherosclerotic lesion development in low-density lipoprotein receptor knockout mice.
Arterioscler Thromb Vasc Biol,
2011 Jan;31(1):67-73.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Chandak PG, Obrowsky S, Radovic B, Doddapattar P, Aflaki E, Kratzer A, Doshi LS, Povoden S, Ahammer H, Hoefler G, Levak-Frank S,
Kratky D:
Lack of acyl-CoA : diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates
atherosclerosis in apolipoprotein E knockout mice.
Biochim Biophys Acta,
2011; 1811(12):1011–1020.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Amor M, Bianco V, Buerger M, Lechleitner M, Vujić N, Dobrijević A, Akhmetshina A, Pirchheim A, Schwarz B, Pessentheiner AR,
Baumgartner F, Rampitsch K, Schauer S, Klobučar I, Degoricija V, Pregartner G, Kummer D, Svecla M, Sommer G, Kolb D, Holzapfel GA,
Hoefler G, Frank S, Norata GD, Kratky D:
Genetic deletion of MMP12 ameliorates cardiometabolic disease by improving insulin sensitivity, systemic inflammation, and atherosclerotic
features in mice.
Cardiovasc Diabetol,
2023; 22(1):327.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Obrowsky S, Chandak PG, Patankar JV, Pfeifer T, Povoden S, Schreiber R, Haemmerle G, Levak-Frank S, Kratky D:
Cholesteryl ester accumulation and accelerated cholesterol absorption in intestine-specific hormone sensitive lipase-null mice.
Biochim Biophys Acta,
2012; 1821(11):1406–1414.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Obrowsky S, Chandak PG, Patankar JV, Povoden S, Schlager S, Kershaw EE, Bogner-Strauss JG, Hoefler G, Levak-Frank S, Kratky D:
Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling.
J Lipid Res,
2013; 54(2):425–435.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Han YH, Onufer EJ, Huang LH, Sprung RW, Davidson WS, Czepielewski RS, Wohltmann M, Sorci-Thomas MG, Warner BW, Randolph GJ:
Enterically derived high-density lipoprotein restrains liver injury through the portal vein.
Science,
2021; 373(6553):eabe6729.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
|
|






![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)