


The MET-FLAM Faculty
Personal information: |

| |||||||||||||||||||||||||
| Name: | Christian WADSACK | |||||||||||||||||||||||||
| Univ.-Prof. Dr. rer. nat. (PhD) | ||||||||||||||||||||||||||
| Group leader of the Placenta Lab,
Department of Obstetrics and Gynecology | ||||||||||||||||||||||||||
|
Department of Obstetrics and Gynecology, Medical University of Graz,
Auenbruggerplatz 14, A-8036 Graz;
| ||||||||||||||||||||||||||
| [Team] [Personal] | ||||||||||||||||||||||||||
| [0000-0002-5589-8642] | ||||||||||||||||||||||||||
| [semanticscholar] | ||||||||||||||||||||||||||
Scientific Interests:We are a group of researchers at the Medical University of Graz fascinated with the human placenta, an organ formed especially for the purpose of pregnancy. It acts both as a connection and a barrier between mother and baby and its correct development and function are essential to successful pregnancy, and health and well-being of mother and baby. Each of us once grew their own placenta while in the mother's womb, and current research suggests that the first thousand days from pregnancy to the second birthday of a child are a window of opportunity to build a healthy foundation for children's later life. In addition, pregnancy complications stemming from placenta dysfunction can have consequences for the mother's health in the end. Therefore, placental research has an impact on all of us, but especially on future generations.In our research, we investigate pregnancy associated factors, placental physiology, and placental function in healthy and compromised pregnancies. Therefore, we have established a panel of cell and tissue culture models and use functional assays, in combination with innovative molecular analyses to address our research questions. | ||||||||||||||||||||||||||
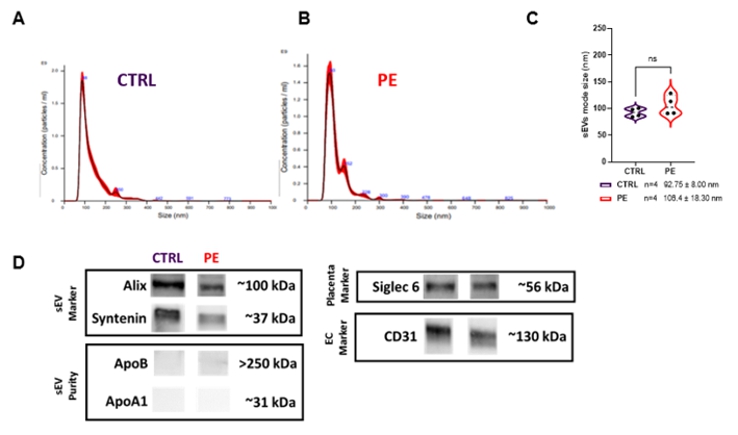
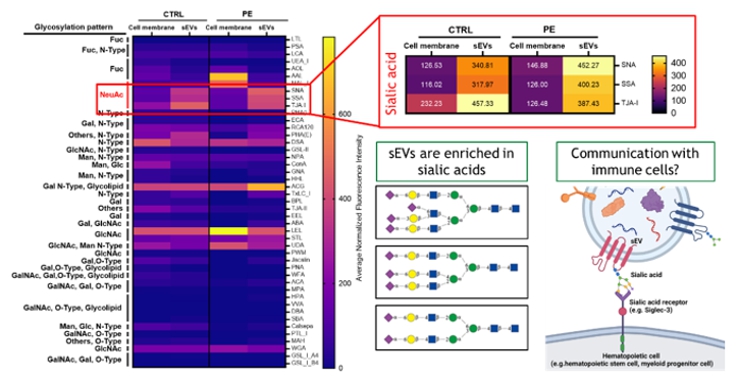
Proposed Dissertation Topic:Extracellular placental vesicles modulate inflammatory responses of fetal immune cellsBackground: Pregnancy represents a unique challenge to the maternal-fetal immune interface. It requires a balance between immunosuppression, which is essential to maintain a semi-allogeneic fetus, and proinflammatory host defenses to protect the maternal-fetal interface from invading microorganisms. Exaggerated inflammatory responses to metabolic derangements in the mother may be critical for an adequate fetal development as the placenta transmits such adverse signals. Extracellular vesicles (EVs) are membrane-bound complexes released by cells and are potent signaling modulators of the immune system under both physiological and pathological conditions [1]. The placenta and the maternal immune system communicate via the release of soluble factors such as cytokines and through components carried by EVs [2]; such released EVs have immunomodulatory roles during pregnancy [3]. However, how the placenta communicates with the fetal circulation and whether EVs act as carriers is largely unknown. We have previously shown that maternal lipoproteins differ from fetal particles in their composition, function, and interaction with the placental barrier [4]. In this context, we suggest that placental EVs derived from endothelial cells (ECs) may play distinct roles in modulating fetal metabolism. Hypothesis and objectives: Preeclampsia (PE), a severe and frequent disorder in pregnancy, is often accompanied by a reduced tolerance of the semi-allogenic fetus and may lead to unfavorable outcomes. We hypothesize that PE placenta-derived ECEVs cause an exacerbated inflammatory response in fetal immune cells compared to normal ECEVs, thereby inducing a myriad of cellular and metabolic alterations in the unborn. In particular, the student will examine (i) whether there are differences in the ECEVs protein / lipid / mRNA cargo between normal and PE-derived placentae; (ii) whether cord-blood-derived CD34+ immune cells interact differently with ECEVs, and (iii) whether ECEVs isolated from PE placentae might trigger an altered inflammatory response in fetal immune cells compared to those derived from normal placentae.
Methods and approaches:
In the 1st year, the PhD candidate will be instructed in the isolation and characterization of primary human ECs from placental tissue
using proteolytic enzymes and by immunofluorescence analyses, respectively. The student will be trained to isolate ECEVs from
supernatants of
Pitfalls and alternative approaches:
The followings are the estimated risk of the proposed project and the contingency plans for them:
Involved Faculty members:
Christian Wadsack (PI), Gunther Marsche and Eva Sturm (activation assay for
neutrophils / monocytes, eosinophils and basophils), Ákos Heinemann (inflammatory lipid
mediator profiling), Stefano Angiari ( Facilities: The Wadsack lab is an interdisciplinary team of two post docs, four PhD candidates, two study nurses and one technician. Our lab has access to maternal / fetal blood and placental samples including all clinical information of more than 3,500 normal and compromised deliveries. The wet lab is equipped with facilities for cell culture, immunofluorescence, western blot, real-time qPCR that enables isolating, characterizing and culturing all types of primary cells in the placenta. Furthermore, we have established the ex vivo dual placental perfusion model which allows us to perform maternal to fetal transfer studies and to isolate placental extracellular vesicles distinctly. The lab is equipped with ECIS system including a versatile pump system (Ibidi) to monitor barrier function of cells and for the stimulation of physiological systems with defined shear stress, mimicking blood vessels, endothelial cells under flow, rolling and adhesion assays perfusion of cells, spheroids and organoids for optimal nutrition. OMICS approaches are performed in a long-standing cooperation with core facilities of the Med Uni Graz and with Karl Kashofer, head of the Molecular Pathology lab at our University. Preparatory Findings:
| ||||||||||||||||||||||||||
|
References:
| ||||||||||||||||||||||||||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)