


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Gunther MARSCHE | |
| Assoc. Prof. Dr. rer. nat. (PhD) | ||
| Associate Professor, Head of Research Unit | ||
|
Otto Loewi Research Centre, Division of Pharmacology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Research Centre] [Section] [Personal] | ||
| [0000-0002-2422-5381] | ||
| [semanticscholar] | ||
Scientific Interests:It is increasingly recognized that lipid and lipoprotein pathways play an important role in immunity and infection [1]. Of particular interest is high-density lipoprotein (HDL), for which the most convincing data have been found. Research conducted in animal models has demonstrated the importance of functional changes in the metabolic development of HDL through the acute phase response [2]. In humans, several observational studies have shown changes in lipids and lipoproteins in patients with severe infections, while population-based epidemiological studies have found associations between specific lipid and lipoprotein classes and the risk of severe infection [3].HDL has long been associated with epidemiological research, as an inverse correlation was initially found between HDL cholesterol concentration in the blood and cardiovascular disease, earning it the nickname “good cholesterol”. However, subsequent randomized trials and genetic evidence have shown that circulating HDL cholesterol does not play a fundamental role in atherothrombotic cardiovascular disease [4]. During the acute phase of the response, the concentration, function, and structure of HDL particles change, and several lines of evidence suggest a possible biological role for these altered HDL particles in infection and inflammation. Thus, despite its unclear biological role, HDL is conserved across different biological species; however, HDL has been shown to have anti-inflammatory characteristics and may play a protective role in severe inflammation. We and others have found that low levels of HDL are associated with poor outcomes in patients with liver cirrhosis, sepsis or Covid-19 infection [5, 6, 7]. HDL has been shown to have a protective effect on endothelial cells, by reducing their susceptibility to inflammation and oxidative stress. HDL can also bind to and neutralize bacterial toxins, preventing them from causing further damage in the body. Moreover, HDL can stimulate the production of anti-inflammatory cytokines and inhibit the production of pro-inflammatory cytokines, further suppressing the inflammatory response that occurs in sepsis. HDL has also been shown to have antimicrobial properties, as it can directly inhibit the growth of certain bacteria. However, further research is needed to fully understand the mechanisms underlying HDL’s protective effects and to develop effective treatments that can harness these benefits. Inflammation alters many of the functional properties of HDL, as we and others have observed in HDL isolated from patients suffering from chronic inflammatory diseases including chronic kidney disease [8], cirrhosis [9], or psoriasis [10], to name a few examples. Therefore, further studies are needed to better understand this new concept of HDL dysfunction, which is clearly associated with poor outcome. A better understanding of the metabolism, composition and function of HDL particles in sepsis should contribute to finding new therapeutic approaches in severe inflammatory diseases. These findings support the therapeutic potential of reconstituted HDL or HDL-mimetic peptide infusion during severe inflammation. Preliminary data from first experimental studies using reconstituted HDLs or HDL mimetic peptides are promising, as replenishing HDL with synthetic HDL has been found to have multiple protective effects against sepsis in mice [11]. | ||
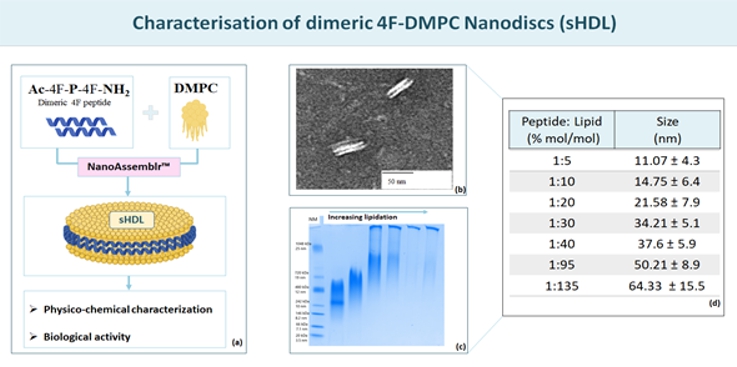
Proposed Dissertation Topic:Understanding high-density lipoprotein metabolism and function in acute inflammation and sepsisBackground: Lipopolysaccharide (LPS) is a potent endotoxin responsible for the pathophysiological symptoms of infection. Its association with plasma lipoproteins, particularly high-density lipoprotein (HDL) with its high binding capacity, suggests a critical role for lipoproteins in LPS detoxification. In addition to LPS sequestration, HDL modulates the function of immune and endothelial cells, in both innate and adaptive immunity. Serum lipoprotein concentrations decrease drastically in acute inflammation and sepsis, and low HDL levels in the intensive care unit are strongly associated with mortality, suggesting a causal relationship. The pathophysiology of the changes in HDL metabolism that occur in the early phase of sepsis remains to be elucidated. Elucidation of these mechanisms holds immense potential for improved prevention and treatment strategies for acute inflammation and sepsis. Hypothesis and objectives: The major goals of the project are to understand how severe inflammation influences the metabolism of HDL and affects its composition and function, particularly its capacity to neutralize LPS, to suppress monocyte / neutrophil effector responses and platelet activation, and to promote endothelial barrier function. The pro-resolving effects of synthetic reconstituted HDL (rHDL) on primary blood monocyte and neutrophil effector responses, endotoxin inactivation, and endothelial function will be tested. In addition, rHDL will be applied to models of acute lung inflammation to evaluate its potential beneficial properties. Methods and approaches: The PhD candidate will be trained in isolating HDL from human serum and in purifying leukocytes and platelets from peripheral blood. The PhD candidate will examine whether HDL isolated from plasma of septic patients [5] is dysfunctional regarding (i) binding of LPS, (ii) suppression of neutrophil effector responses, such as CD11b upregulation, degranulation, phagocytosis, chemotaxis, and downstream signalling, (iii) inhibition of neutrophil-endothelial adhesion under flow, and (iv) promotion of endothelial barrier function by electrical impedance measurements. In addition, the PhD candidate will perform native gel electrophoresis and western blotting to analyse the structure and composition of HDL, flow cytometry to determine the expression of endothelial receptors and adhesion molecules, and multiplex ELISA to measure cytokine release (1st and 2nd year). In the 3rd and 4th year, the PhD candidate will learn to produce and characterize synthetic reconstituted HDL formulations as shown in preparatory data below. The student will test whether rHDL can compensate for the loss of function of HDL during severe inflammation. The PhD candidate will then assess whether systemic application of rHDL dampens LPS-induced lung pathology in mice, decreases neutrophil influx into the airways, lowers myeloperoxidase activity in lung tissue, reduces cytokine levels, and improves lung function and survival. Pitfalls and alternative approaches: Severely septic patients often have severely decreased HDL levels, making isolation and functional characterization challenging. In addition, isolation techniques such as ultracentrifugation can potentially alter HDL functionality. To potentially obtain less modified HDL particles, we will use immunoaffinity chromatography to purify HDL subspecies. In addition, reconstituting the full complexity of functional HDL components and their interactions in reconstituted HDL may be difficult. Therefore, we will investigate the use of synthetic peptides that mimic functional domains of HDL as an alternative to rHDL. Involved Faculty members: Gunther Marsche (PI), Eva Sturm and Julia Kargl (mouse models and lung function testing), Dagmar Kratky (intestinal derived HDL), Ákos Heinemann (immune-cell effector responses). International Collaborations: Christina Christoffersen (University of Copenhagen, DEN), Mounir Tarek (Université de Lorraine, FRA), Burkhard Bechinger (University of Strasbourg, FRA), Graziella Eliza Ronsein (University of São Paulo, BRA). Facilities: Our current research team comprises three PhD candidates, one post-doctoral researcher, and two part-time research assistants. Our department provides the basic laboratory facilities such as cell culture, fast protein liquid chromatography, flow cytometry, radionuclide laboratory. The Cellix platform allows to perform experiments on endothelial cells using microfluidic technology. Equipment for lung function tests on mice are available in our department. We continuously isolate all types of human peripheral blood leukocytes from blood donors in our department. Our cooperation partners on campus offer mass spectrometry services and metabolomics. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)