|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Vanessa STADLBAUER-KÖLLNER
|
| Acad. Degree:
| Univ.-Prof. Priv.-Doz. Dr. med. univ. (MD), MBA
|
| Current Position:
| Head, Research Unit “Translational Microbiome Modulation”,
Medical University of Graz
|
| Contact Details:
|
Department of Gastroenterology and Hepatology, Medical University of Graz,
Auenbruggerplatz 15, A-8036 Graz;
phone: +43 316 385 82282,
✉ e-mail
|
| Websites:
|
[Personal]
|
| ORCID:
|
[0000-0000-0000-0000]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
The research lab of Vanessa Stadlbauer-Köllner works in the field of translational medicine, combining immunology, microbiome research
and biomarker research with clinical medicine. We described neutrophil dysfunction in liver cirrhosis and showed that impaired neutrophil
phagocytosis and dysregulated ROS production is association with infections and mortality in cirrhosis. Bacterial metabolites,
hepatitis C infections as well as bile acids are associated with the development of neutrophil dysfunction. Removal of bacterial
products; elimination of hepatitis C infection and microbiome modulation via probiotics can improve neutrophil dysfunction
[1–8].
We also described factors that influence microbiome composition in liver cirrhosis (liver disease etiology, severity, drug intake,
inflammation and nutritional status). We found a strong association of proton pump inhibitor use and “oralization” (the
occurrence of oral bacteria Veillonella parvula and Streptococcus salivarius in the gut microbiome) with inflammation,
complications and mortality. Besides medical acid suppression, also reduction of stomach acid production due to surgical resection causes
this oralization. We investigated the effect of microbiome modulation via probiotics as possible strategy to improve microbiome composition
in cirrhosis. [9–12] We further demonstrated interactions between disease, drug intake and
the composition and function on the gut microbiome in patients with liver cirrhosis, dementia, chronic kidney disease, polycystic ovary
syndrome and secondary sclerosing cholangitis in critically ill patients. [11, 13–16]
We further discovered that alcohol intake is associated with a steep and rapid increase in FGF21 levels and contributed to the understanding
the role of FGF21 in the regulation of water intake [17, 18].
We currently have a strong research interest in sarcopenia (muscle loss) in liver cirrhosis and we recently described the role of secondary
bile acids, which are transformed from primary bile acids by the gut microbiome in sarcopenia in cirrhosis. We also work on clinical
sarcopenia projects where we aim to improve diagnostics by using machine learning tools and to predict prognosis with a variety of
traditional and non-traditional biomarkers [19, 20]. We aim to deepen the knowledge on the
pathophysiology of the disease as well as the peculiarities of sarcopenia in liver diseases and to identify novel therapeutic targets. We
currently perform the first interventional study treat muscle loss with a microbiome modulating strategies.
|
Proposed Dissertation Topic:
The impact of bacterial bile acid metabolism on gut permeability, inflammation and muscle-cell function in cirrhosis and sarcopenia
Background:
The loss of muscle mass, quality and strength (sarcopenia) is a common problem of older age, especially in people with chronic diseases
such as liver cirrhosis. Sarcopenia impairs quality of life and increases mortality. Decreased protein synthesis and increased protein
degradation mediated by inflammation contribute to sarcopenia in cirrhosis. However, the starting point of this negative cascade of events
has not been fully elucidated and therefore, to date, no specific therapy is available to treat or prevent sarcopenia. We have recently
identified distinct changes in secondary bile acid abundance and composition being associated with sarcopenia. Furthermore, we have shown
that bile acids induce neutrophil ROS production ex vivo, indicating a link between the gut microbiome, bile acids, and
inflammation in cirrhosis.
Hypothesis and objectives:
We hypothesize that changes in microbial bile acid metabolism play a key role in the development of sarcopenia in cirrhosis. The PhD
student will dissect the effects of cirrhosis and sarcopenia on the transformation of primary to secondary bile acids in the gut microbiome
using a patient-derived in vitro cultivated microbiome model and will identify in silico and in vitro differences
in bile acid transformation pathways in stool microbiomes of cirrhotic patients with and without sarcopenia. Furthermore, the student will
investigate the effects of altered bile acid composition on gut permeability and inflammation as well as muscle cell function in
ex vivo / in vitro cell culture model systems.
Methods and approaches:
The PhD candidate will learn to use an anaerobic bioreactor system developed for long-term culture of the human stool microbiome. The student
will evaluate bile acid profiles by LC-MS-MS and perform metagenomics analysis (1st year). The student will use an in vitro
model of the intestinal barrier (Caco-2 cells, assessment of transepithelial resistance, expression of tight junction and
adherens junction proteins) and an ex vivo innate immune model, in which neutrophils and peripheral blood mononuclear cells will
be challenged with different bile acid compositions or the supernatant of in vitro anaerobic microbiome cultures to assess the
production of reactive oxygen species, calprotectin, and cytokines (2nd and 3rd year). Adult human skeletal muscle cells will be cultivated
by the student and challenged with different bile acid compositions, and a comprehensive cell biology qPCR array for human skeletal muscles
will be performed to characterize muscle-cell contractility, development and proliferation, differentiation, muscle atrophy and dystrophy
(4th year). The student will be trained to apply advanced biostatistical and bioinformatics tools to analyze complex multi-omics
datasets obtained from the experimental work.
Pitfalls and alternative approaches:
The followings are the estimated risk of the proposed project and the contingency plans for them:
| Risk description:
| Mitigation:
|
Unavailability of patient material (low risk)
- Established clinical network and clinical study coordination structure within the research unit of the PI
No differences are detectable in bile acid transformation between cirrhosis and sarcopenia (medium risk)
- Metagenomics analysis beyond bile acid metabolism, identification of additional pathways of interest, analysis of potential additional
microbial metabolites on muscle cells and innate immune cells
Unavailability of adult human skeletal muscle cells (medium risk)
- Training of the PhD candidate at the “Center of Myology” in Paris, France, in the group of Professor Trollet and Professor Mouly
(contact established), use of established cell lines or use of rodent muscle cells
| |
| |
| |
Involved Faculty members:
Vanessa Stadlbauer-Köllner (PI), Julia Kargl (innate immune model), Aitak Farzi
(intestinal permeability).
International Collaborations:
Paul O'Toole School of Microbiology, APC Microbiome Ireland, University College Cork (microbiome modulation).
Facilities:
Our lab has developed and fully established the in vitro models for neutrophil function, the in vitro model of the
gut microbiome (Figure 1) and an in vitro model of the gut barrier (Figure 2).
The lab is located at the Zentrum für Medizinische Grundlagenforschung (ZMF).
At ZMF the team has also access to cell culture facilities, flow cytometry, imaging, and all general lab methods
(e. g. western blot, photometry, qPCR). Access to LC-MS/MS and untargeted metabolomics techniques as well
as microbiome sequencing is available through the ZMF core facilities and our collaboration partners. For translational research, access to
patient data and clinical samples is crucial, which is available at the Department of Gastroenterology and Hepatology and through the study
coordination team of the research group.
Preparatory Findings:
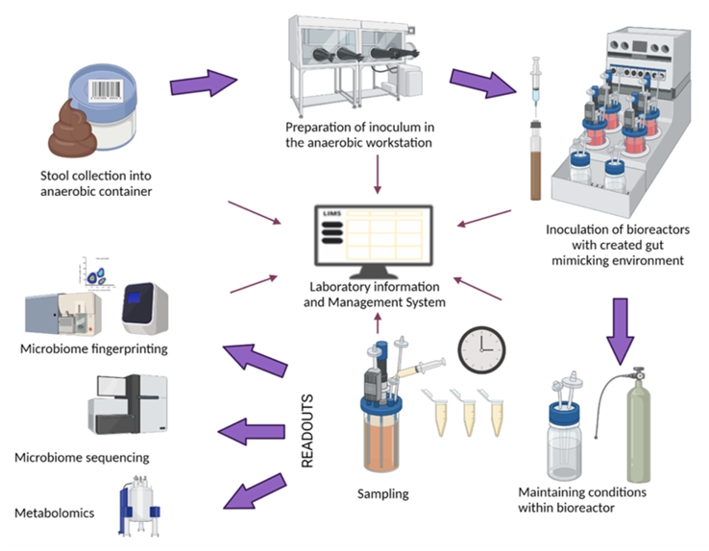

Figure 1. Setup of the in vitro gut microbiome model.
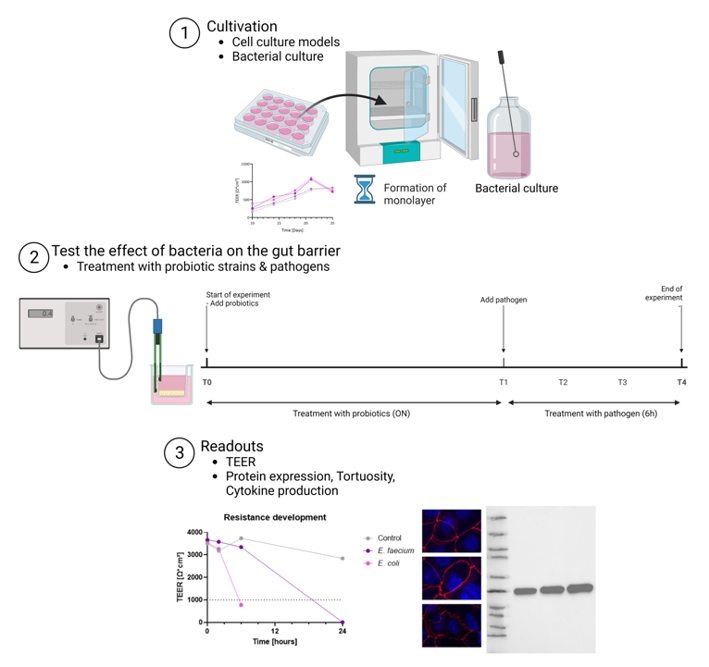
Figure 2. Setup of the gut barrier model.
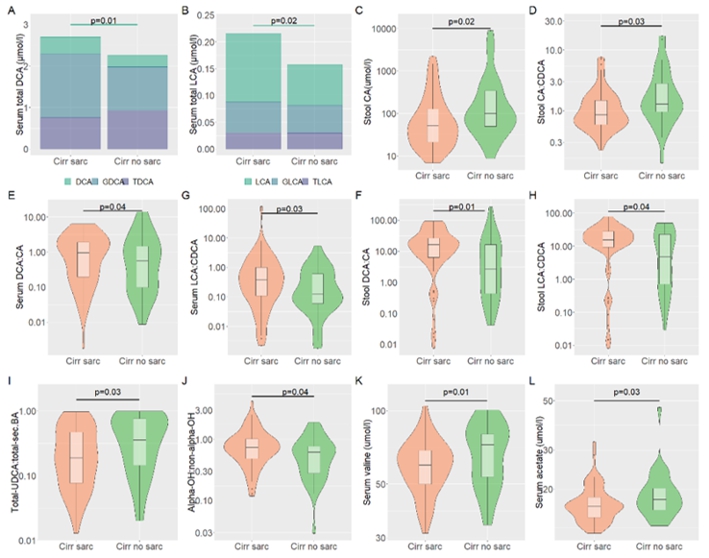
Figure 3. Preliminary data on differences in bile acid composition in patients with liver cirrhosis with and without sarcopenia.
Altered secondary and primary BAs between cirrhotic patients with and without sarcopenia (A–C). Shift from classical
to the alternative pathway between cirrhotic patients with and without sarcopenia (D). Altered BAs transformation from primary to
secondary BAs between cirrhotic patients with and without sarcopenia (E–H). Altered BAs pool hydrophilicity status
between the cirrhotic patients with and without sarcopenia (I). Altered BA 12α-hydroxylation between cirrhotic patients with
and without sarcopenia (J). Altered gut microbiome-derived metabolites, BCAA, and SCFA between cirrhotic patients with and without
sarcopenia (K–L).
|
|
References:
-
Balazs I, Horvath A, Leber B, Feldbacher N, Sattler W, Rainer F, Fauler G, Vermeren S, Stadlbauer V:
Serum bile acids in liver cirrhosis promote neutrophil dysfunction.
Clin Transl Med,
2022; 12(2):e735.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Leber B, Balázs I, Horvath A, Posch A, Streit A, Spindelböck W, Feldbacher N, Stiegler P, Stauber RE, Rechberger GN,
Kollroser M, Sattler W, Nusshold C, Stadlbauer V:
Direct acting antiviral therapy rescues neutrophil dysfunction and reduces hemolysis in hepatitis C infection.
Transl Res,
2021; 232:103–114.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Horvath A, Leber B, Feldbacher N, Tripolt N, Rainer F, Blesl A, Trieb M, Marsche G, Sourij H, Stadlbauer V:
Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality
of life in diabesity: a randomized, double-blind, placebo-controlled pilot study.
Eur J Nutr,
2020; 59(7):2969–2983.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R:
Evidence of neutrophil functional defect despite inflammation in stable cirrhosis .
J Hepatol,
2011; 55(3):574–581.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jürgens G, Hallström S, Jalan R:
Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis.
Am J Physiol Gastrointest Liver Physiol,
2009; 296(1):G15–G22.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, Pitsillides AA, Jalan R:
Ammonia impairs neutrophil phagocytic function in liver disease.
Hepatology,
2008; 48(4):1202–1212.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R:
Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis.
J Hepatol,
2008; 48(6):945–951.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R:
Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome.
Hepatology,
2007; 46(3):831–840.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Horvath A, Bausys A, Sabaliauskaite R, Stratilatovas E, Jarmalaite S, Schuetz B, Stiegler P, Bausys R, Stadlbauer V, Strupas K:
Distal Gastrectomy with Billroth II Reconstruction is Associated with Oralization of Gut Microbiome and Intestinal Inflammation:
A Proof-of-Concept Study.
Ann Surg Oncol,
2021; 28(2):1198–1208.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Horvath A, Leber B, Feldbacher N, Steinwender M, Komarova I, Rainer F, Blesl A, Stadlbauer V:
The effects of a multispecies synbiotic on microbiome-related side effects of long-term proton pump inhibitor use: A pilot study.
Sci Rep,
2020; 10(1):2723.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Stadlbauer V, Komarova I, Klymiuk I, Durdevic M, Reisinger A, Blesl A, Rainer F, Horvath A:
Disease severity and proton pump inhibitor use impact strongest on faecal microbiome composition in liver cirrhosis.
Liver Int,
2020; 40(4):866–877.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Horvath A, Rainer F, Bashir M, Leber B, Schmerboeck B, Klymiuk I, Groselj-Strele A, Durdevic M, Freedberg DE, Abrams JA, Fickert P,
Stiegler P, Stadlbauer V:
Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis.
Sci Rep,
2019; 9(1):12000.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Blesl A, Jüngst C, Lammert F, Fauler G, Rainer F, Leber B, Feldbacher N, Stromberger S, Wildburger R, Spindelböck W,
Fickert P, Horvath A, Stadlbauer V:
Secondary Sclerosing Cholangitis in Critically Ill Patients Alters the Gut-Liver Axis: A Case Control Study.
Nutrients,
2020; 12(9):2728.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Stadlbauer V, Engertsberger L, Komarova I, Feldbacher N, Leber B, Pichler G, Fink N, Scarpatetti M, Schippinger W, Schmidt R, Horvath A:
Dysbiosis, gut barrier dysfunction and inflammation in dementia: a pilot study.
BMC Geriatr,
2020; 20(1):248.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Stadlbauer V, Horvath A, Ribitsch W, Schmerböck B, Schilcher G, Lemesch S, Stiegler P, Rosenkranz AR, Fickert P, Leber B:
Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis.
Sci Rep,
2017; 7(1):15601.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR, Gorkiewicz G, Stadlbauer V,
Obermayer-Pietsch B:
Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with
Polycystic Ovary Syndrome (PCOS): A Pilot Study.
PLoS One,
2017; 3;12(1):e0168390.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Wagner-Skacel J, Horvath A, Grande P, Wenninger J, Matzer F, Fazekas C, Mörkl S, Meinitzer A, Stadlbauer V:
Association of fibroblast growth factor 21 with alcohol consumption and alcohol liver cirrhosis.
Neuropsychiatr,
2021; 35(3):140–146.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Song P, Zechner C, Hernandez G, Cánovas J, Xie Y, Sondhi V, Wagner M, Stadlbauer V, Horvath A, Leber B, Hu MC, Moe OW, Mangelsdorf DJ,
Kliewer SA:
The Hormone FGF21 Stimulates Water Drinking in Response to Ketogenic Diet and Alcohol.
Cell Metab,
2018; 27(6):1338–1347.e4.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Schweighofer N, Colantonio C, Haudum CW, Hutz B, Kolesnik E, Mursic I, Pilz S, Schmidt A, Stadlbauer V, Zirlik A, Pieber TR,
Verheyen N, Obermayer-Pietsch B:
DXA-Derived Indices in the Characterisation of Sarcopenia.
Nutrients,
2021; 14(1):186.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Traub J, Bergheim I, Eibisberger M, Stadlbauer V:
Sarcopenia and Liver Cirrhosis-Comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019.
Nutrients,
2020; 12(2):547.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
|
|








![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)