


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Eva STURM | |
| Assoc. Prof. Dr. rer. nat. (PhD) | ||
| Associate Professor at the Otto Loewi Research Center,
Division of Pharmacology | ||
|
Otto Loewi Research Centre, Division of Immunology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Research Centre] [Pharmacology] [Team] [Personal] | ||
| [0000-0003-4898-884X] | ||
| [semanticscholar] | ||
Scientific Interests:Our research group’s interests lie primarily in the field of chronic inflammatory diseases with a special focus on eosinophilia-associated respiratory and gastrointestinal diseases such as bronchial asthma and eosinophilic esophagitis [1–10]. In recent years we have elucidated several molecular disease mechanisms and signaling cascades and demonstrated their therapeutic potential with a translational approach combining in&nnbsp;vitro assays, experimental mouse models and analysis of patient samples.
Janus kinases are crucial components of cytokine signaling pathways which are involved in the regulation of many cellular functions.
Cytokine-induced persistent or dysregulated Janus kinase signaling, however, is maladaptive and contributes to many inflammatory diseases.
We observed that targeting the JAK1/2 pathway with the JAK inhibitor Baricitinib represents a promising therapeutic strategy for
eosinophilic inflammation as observed in severe eosinophilic asthma [11]. In a follow-up project we investigate the
therapeutic potential of JAK inhibitors in
Apolipoproteins (APO) such as | ||
Proposed Dissertation Topic:Novel tautomerase inhibitors in inflammatory lung diseases
Background:
Macrophage migration inhibitory factor (MIF) is elevated in asthma patients and positively correlates with blood eosinophils, but is negatively
associated with lung function indices. MIF functions as a nuclease and participates in the induction of parthanatos, a type of poly
(ADP-ribose) polymerase-induced cell death. MIF also acts as a regulator of the NLRP3 inflammasome and has enzymatic activities, such as
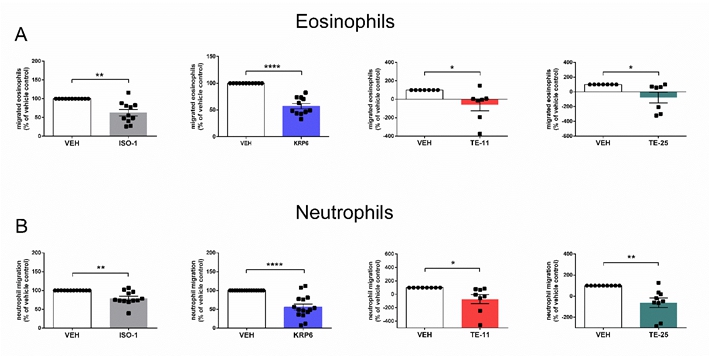
tautomerization of phenylpyruvate and Hypothesis and objectives: We aim to elucidate the therapeutic potential of novel inhibitors targeting the enzymatic activities of MIF in patients with severe eosinophilic asthma and respective mouse models. We plan to investigate how MIF and MIF inhibitors influence (i) effector function, (ii) glucose homeostasis and (iii) autophagy in peripheral blood and induced sputum leukocytes from controls and asthmatic patients. (iv) We will elucidate MIF-associated signaling cascades and how they can be modulated with respective inhibitors, and (v) prove the in vivo relevance of these approaches in established murine models of eosinophilic lung inflammation. Methods and approaches: The PhD candidate will be trained in isolating leukocyte subsets from peripheral blood from healthy donors and patients (asthma and acute respiratory distress syndrome) by negative magnetic selection and cell sorting. The expression of CD74 and NLRP3 will be quantified by flow cytometry, qPCR, western blotting, and fluorescence microscopy. MIF levels and other cytokines in serum, BALF and tissue homogenates will be determined by ELISA, lipid mediators will be measured by mass spectrometry. Functional responses of leukocytes will be investigated in flow cytometric assays of shape change, integrin up-regulation, respiratory burst, and chemotaxis. Autophagic activity and energetic metabolism will be determined using SCENITH and Seahorse analyses (1st and 2nd year). Signaling pathways will be explored by live-cell Ca2+ imaging, flow cytometry, and western blotting. For silencing and overexpression studies, bone-marrow-derived mouse eosinophils and monocyte-derived macrophages will be used. Moreover, the student will be trained in established experimental mouse models for LPS-induced acute lung injury and allergen-induced airway inflammation. Lung tissue will be analyzed by immunohistochemistry and in situ hybridization. Lung function testing will be performed by using the flexiVent platform (3rd and 4th year). MIF levels and other cytokines in serum, bronchoalveolar lavage fluid, sputum and tissue homogenates will be determined by ELISA, lipid mediators will be measured by mass spectrometry. Pitfalls and alternative approaches: Our laboratory has many years of experience with in vitro and in vivo models of lung inflammation. Therefore, we do not expect any technical pitfalls here. However, in the unexpected case, that we do not observe any effects of the respective MIF inhibitors in the planned experiments, we will alternatively (i) monitor other inflammatory cells such as monocytes, macrophages, neutrophils and lymphocytes in blood and sputum of patients as well was in BALF and lung tissue of mice. As MIF also seems to influence epithelial barrier function and tissue remodeling, (ii) we will explore the impact of MIF and MIF inhibitors on energy metabolism, cytokine release and epithelial barrier function in primary bronchial epithelial cells (PBECs) from healthy controls and patients with severe eosinophilic asthma. (iii) Further, organotypic precision-cut lung slices (PCLS) are a well-established model for allergic pulmonary inflammation. Therefore, the beneficial effects of MIF inhibitors on cytokine release, markers of collagen formation and degradation, as well as fibronectin will be studied in activated human and mouse PCLS. (iv) Finally, we will take advantage of publicly available transcriptomic data sets and investigate the expression of MIF-related pathways in samples from healthy and asthmatic individuals. Involved Faculty members: Eva Sturm (PI), Julia Kargl (multi-color flow cytometry and cell sorting), Ákos Heinemann (leukocyte functional assays), Stefano Angiari (cell signaling assays and Seahorse analysis) and Martin Stradner (SCENITH: autophagy and energetic metabolism). International Collaborations: Balázs Radnai (Pécs, HU), Hannah Durrington (Manchester, UK), Robert Gurke (Frankfurt, DE). Facilities: Our team currently consists of two PhD candidates and two technicians. Our laboratory is located on the newly opened basic research campus of the university, just opposite the university hospital. Our division provides the required laboratory and office space, secretarial assistance, and basic laboratory facilities, such as cell culture, flow cytometry, immunofluorescence microscopy and more. Specific equipment for mouse lung function testing and endothelial functional assays (interaction of endothelial cells and immune cells; endothelial barrier function) are also available in our lab. We are constantly isolating fresh human peripheral blood leukocytes from blood donors. Seahorse technology, metabolomics and mass spectrometry are also available on the campus through collaboration partners. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)