|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Julia KARGL
|
| Acad. Degree:
| Assoc. Prof., PhD
|
| Current Position:
| Group Leader 'Cancer Immunology'
Otto Loewi Research Center, Division of Pharmacology
|
| Contact Details:
|
Otto Loewi Research Centre, Division of Immunology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
phone: +43 316 385 74105,
✉ e-mail
|
| Websites:
|
[Otto Loewi Research Centre]
[Pharmacology]
[Team]
[Personal]
|
| ORCID:
|
[0000-0002-0870-0816]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
The scientific interests of our group are at the intersection of pharmacology and immunology. Our research is focused on the role of the
immune system in lung cancer and how the immune system can be exploited to detect tumors earlier and fight them better. Our international
team investigates the immune cell composition in lung cancer and the role of immunosuppressive cells — an important
aspect in future development of immune-based drugs, also called tumor immunotherapy. Selected key publications highlight our contribution
to the field of tumor immunology and the importance of understanding the immune microenvironment in cancer patients. This includes in-depth
phenotyping of NSCLC patients, the contribution of COPD and smoking on immunotherapy outcome and that neutrophil abundance in the tumor
microenvironment can predict checkpoint inhibitor therapy failure [1– 4]. Since I have
started my independent group, we have defined distinct low-density neutrophil markers and a novel mechanism of neutrophil polarization in
the tumor microenvironment inducing arginase 1 expression [5, 6]. Further, we study the
importance of neutrophil derived enzymes (neutrophile elastase and myeloperoxidase) in modulating lung cancer growth and lung fibrosis
(5, 7 – 9] and the role of cannabinoid receptors,
GPR55 and their ligands in health and disease [10 – 16].
Recently, our research interests expanded in the field of cellular metabolism, which can affect immune cell function and promote disease
pathology. Understanding the interplay between the immune microenvironment and cellular metabolism in lung diseases will be important for
the development of effective therapies.
|
Proposed Dissertation Topic:
Addressing metabolic states in neutrophil subsets in lung disease
Background:
Neutrophils are the first line of host defense against bacteria and critically regulate immunity. The relevance of distinct neutrophil
subsets and cellular plasticity has been described in chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF)
and lung cancer. Cellular metabolism plays an essential role in the function and plasticity of immune cells. Neutrophils have long been
considered to solely depend on glycolysis. However, recent studies have shown that the TCA cycle, oxidative phosphorylation (OXPHOS), the
pentose phosphate pathway (PPP), and fatty acid oxidation (FAO) also play a role in neutrophil function and are associated with distinct
phenotypes. Migrating neutrophils in early cancer stages exhibit enhanced OXPHOS and glycolysis but do not show an immunosuppressive
phenotype. In addition, neutrophils adapt to their local metabolic environment.
Hypothesis and objectives:
We hypothesize that the described neutrophil subsets and polarization states with immunostimulatory (N1) or immunosuppressive (N2)
phenotypes exhibit (i) different cellular metabolic states and (ii) that phenotypic and functional changes are a result of
neutrophil adaptation to the local metabolic environment. The PhD candidate will use established mouse models and has access to clinical
tissue samples to decipher the interplay of metabolic rewiring, neutrophil polarization, and
immunostimulatory / immunosuppressive functions in lung disease.
Methods and approaches:
The recruited PhD candidate will build on published N1 / N2 neutrophil phenotypes and preliminary data
identifying novel neutrophil subsets with distinct phenotypes using in-house high-dimensional flow cytometry screening
(InfinityFlow, 360-plex) in patient tissue samples. The PhD candidate will isolate neutrophil subsets by flow sorting and evaluate
immunostimulatory (N1; T-cell activation, TNFα secretion, epithelial cell cytotoxicity) and immunosuppressive (N2;
T-cell suppression, iNOS, arginase 1, ROS release, epithelial cell growth) phenotypes using established
neutrophil / T-cell and neutrophil / epithelial cell co-culture systems. In addition,
she / he will perform MetaFlux analysis of neutrophil subsets, based on publicly available scRNAseq data from lung cancer
patients which will guide Seahorse and isotope tracing experiments on isolated subsets to determine cellular metabolic states (1st and 2nd year).
The local metabolic environment in healthy, early and advanced disease stages will be quantified by mass spectrometry, and neutrophil
phenotypes will be correlated with identified metabolites (3rd year). Finally, the PhD candidate will manipulate the local metabolic
environment and / or cellular metabolites in vitro and in vivo to affect disease onset
and progression (3rd and 4th year).
Pitfalls and alternative approaches:
This project is challenging; however, it has the potential to unravel the cellular metabolic changes underlying neutrophil polarization and
function in vitro and in vivo. The lab and our collaborators have the necessary expertise in all metabolic and functional
methods required and for all research question we propose at least two methods to ensure data reproducibility. Seahorse experiments require a
large number of cells, which might be challenging to sort out of patient material, if cell numbers are insufficient, we will use flow-cytometry-based
metabolic phenotyping methods including SCENITH-based methods (low risk).
Involved Faculty members:
Julia Kargl (PI), Grażyna Kwapiszewska (models for COPD), Ákos Heinemann
(IPF models), Stefano Angiari and Dagmar Kratky (metabolic phenotyping).
International Collaborations:
A. McGarry Houghton (FredHutch, USA), Mike Davies (University of Copenhagen, DK), Brice Gaudilliere, Stanford, USA).
Facilities:
Our team currently consists of two technicians, one postdoc and five PhD candidates, and we are located on the newly opened basic research
campus of the university, just opposite the university
hospital. Our division provides the required laboratory and office space, secretarial assistance, and basic laboratory facilities, such
as cell culture, flow cytometry, immunofluorescence microscopy, mouse facilities and more. Specific equipment for neutrophil functional
assays, seahorse technology, metabolomics and mass spectrometry are also available in our lab and through collaborations on the campus.
We are constantly isolating fresh human leukocytes, including neutrophils from COPD and lung cancer patients, a valuable resource for this
project.
Preparatory Findings:
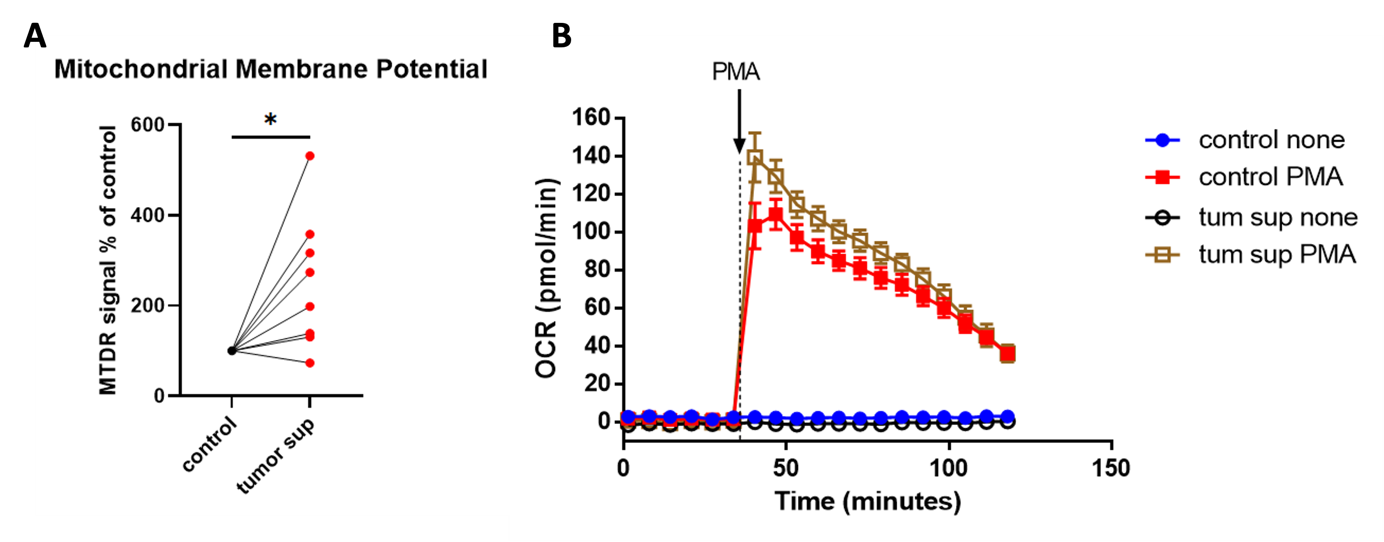
Figure 1.
NSCLC tumor supernatant enhances (A) mitochondrial membrane potential and (B) oxygen consumption in human neutrophils
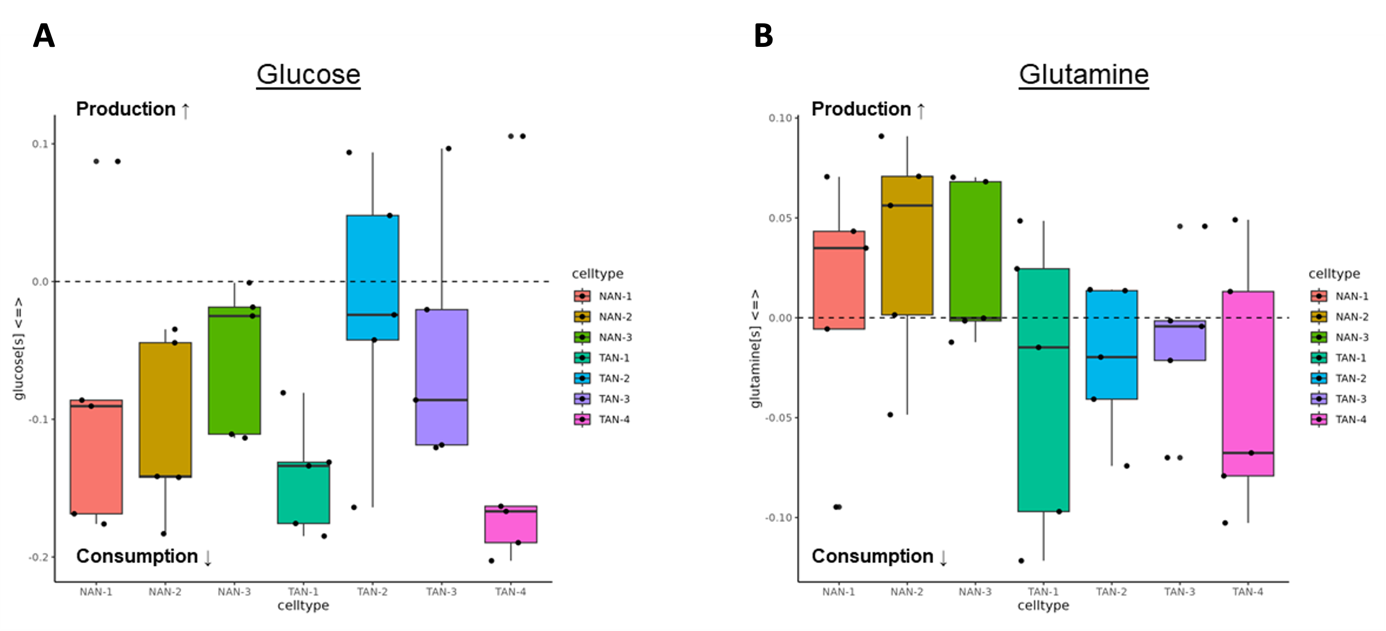
Figure 2. Neutrophils in lung TME show heterogeneity in metabolic flux of (A) glucose and (B) glutamine
(NAN – normal-associated and TAN – tumor-associated neutrophils).
|
|
References:
-
Busch SE, Hanke ML, Kargl J, Metz HE, MacPherson D, Houghton AM:
Lung Cancer Subtypes Generate Unique Immune Responses.
J Immunol,
2016; 197(11):4493–4503.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, Hubbard JJ, Lee SM, Madtes DK, McIntosh MW, Houghton AM:
Neutrophils dominate the immune cell composition in non-small cell lung cancer.
Nat Commun,
2017; 8:14381.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kargl J, Zhu X, Zhang H, Yang GHY, Friesen TJ, Shipley M, Maeda DY, Zebala JA, McKay-Fleisch J, Meredith G, Mashadi-Hossein A, Baik C, Pierce RH, Redman MW, Thompson JC, Albelda SM, Bolouri H, Houghton AM:
Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC.
JCI Insight,
2019; 4(24):e130850.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, Hubbard JJ, Pipavath SNJ, Madtes DK, Houghton AM:
Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non-Small Cell Lung Cancer.
Am J Respir Crit Care Med,
2018; 197(3):325–336.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Zhang H, Zhu X, Friesen TJ, Kwak JW, Pisarenko T, Mekvanich S, Velasco MA, Randolph TW, Kargl J, Houghton AM:
Annexin A2/TLR2/MYD88 pathway induces arginase 1 expression in tumor-associated neutrophils.
J Clin Invest,
2022; 132(22):e153643.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Valadez-Cosmes P, Maitz K, Kindler O, Raftopoulou S, Kienzl M, Santiso A, Mihalic ZN, Brcic L, Lindenmann J, Fediuk M, Pichler M,
Schicho R, Houghton AM, Heinemann A, Kargl J:
Identification of Novel Low-Density Neutrophil Markers Through Unbiased High-Dimensional Flow Cytometry Screening in Non-Small Cell
Lung Cancer Patients.
Front Immunol,
2021; 12:703846.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Gregory AD, Kliment CR, Metz HE, Kim KH, Kargl J, Agostini BA, Crum LT, Oczypok EA, Oury TA, Houghton AM:
Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis.
J Leukoc Biol,
2015; 98(2):143–152.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Metz HE, Kargl J, Busch SE, Kim KH, Kurland BF, Abberbock SR, Randolph-Habecker J, Knoblaugh SE, Kolls JK, White MF, Houghton AM:
Insulin receptor substrate-1 deficiency drives a proinflammatory phenotype in KRAS mutant lung adenocarcinoma.
Proc Natl Acad Sci U S A,
2016; 113(31):8795–8800.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Valadez-Cosmes P, Maitz K, Kindler O, Cosic Mujkanovic N, Lueger A, Raftopoulou S, Kienzl M, Mihalic ZN, Santiso A,
Sarsembayeva A, Brcic L, Lindenmann J, Sattler W, Heinemann A, Schicho R, Marsche G, Houghton AM, Kargl J:
Myeloperoxidase promotes a tumorigenic microenvironment in non-small cell lung cancer.
bioRχiv,
2023.01.28.526014.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Balenga NA, Aflaki E, Kargl J, Platzer W, Schröder R, Blättermann S, Kostenis E, Brown AJ, Heinemann A, Waldhoer M:
GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils.
Cell Res,
2011; 21(10):1452–1469.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Balenga NA, Martínez-Pinilla E, Kargl J, Schröder R, Peinhaupt M, Platzer W, Bálint Z, Zamarbide M,
Dopeso-Reyes IG, Ricobaraza A, Pérez-Ortiz JM, Kostenis E, Waldhoer M, Heinemann A, Franco R:
Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling.
Br J Pharmacol,
2014; 171(23):5387–5406.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kargl J, Andersen L, Hasenöhrl C, Feuersinger D, Stancic A, Fauland A, Magnes C, El-Heliebi A, Lax S, Uranitsch S, Haybaeck J,
Heinemann A, Schicho R:
GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis.
Br J Pharmacol,
2016; 173(1):142–154.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kargl J, Balenga N, Parzmair GP, Brown AJ, Heinemann A, Waldhoer M:
The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55.
J Biol Chem,
2012; 287(53):44234–44248.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kargl J, Balenga NA, Platzer W, Martini L, Whistler JL, Waldhoer M:
The GPCR-associated sorting protein 1 regulates ligand-induced down-regulation of GPR55.
Br J Pharmacol,
2012; 165(8):2611–2619.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kargl J, Brown AJ, Andersen L, Dorn G, Schicho R, Waldhoer M, Heinemann A:
A selective antagonist reveals a potential role of G protein-coupled receptor 55 in platelet and endothelial cell function.
J Pharmacol Exp Ther,
2013; 346(1):54–66.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kienzl M, Hasenoehrl C, Maitz K, Sarsembayeva A, Taschler U, Valadez-Cosmes P, Kindler O, Ristic D, Raftopoulou S, Santiso A,
Bärnthaler T, Brcic L, Hahnefeld L, Gurke R, Thomas D, Geisslinger G, Kargl J, Schicho R:
Monoacylglycerol lipase deficiency in the tumor microenvironment slows tumor growth in non-small cell lung cancer.
Oncoimmunology,
2021; 10(1):1965319.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
|
|






![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)