


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Aitak FARZI | |
| Ass.-Prof. Priv.-Doz. Dr. med. univ. (MD), PhD | ||
| Assistant Professor of Behavioural Pharmacology | ||
|
Otto Loewi Research Centre, Division of Pharmacology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Otto Loewi Research Centre] [Pharmacology] [Team] [Personal] | ||
| [0000-0001-9606-3871] | ||
| [semanticscholar] | ||
Scientific Interests:Gut microbiota-brain signaling is increasingly recognized as an important contributor to brain function and behavior [1]. Our group seeks to characterize the impact of (diet-induced) disturbances of the gut microbiota on peripheral and central metabolism, immune responses, central neurocircuits and behavior [2, 3, 4, 5].
We demonstrated that high fat diet (HFD) feeding induces distinct alterations of intestinal microbiome and behavioral changes indicative
of depression-like behavior as revealed by reduced sociability and anhedonia assessed through sucrose preference. These effects were
paralleled by changes in the metabolome of prefrontal cortex and striatum, changing the relative concentrations of molecules involved in
energy metabolism (
While under obese conditions, antibiotic-induced reduction of the “dysbiotic” gut microbiome attenuates diet-induced
depression-like behavior, we described that antibiotic-induced depletion of the gut microbiota leads to cognitive impairment, specifically
impaired novel object recognition but not spatial memory [9]. This behavioral change was associated with a disruption
of the microbial community in the colon, distinct alterations of the colonic and circulating metabolite profile (lipid species and converted
bacteria-derived molecules) and particular changes of neurochemical brain activity (
Various bacterial metabolites including SCFAs are suggested to exert positive effects on brain function through multiple mechanisms. In
addition, the gut microbiota is a rich source of microbe-associated molecular patterns (MAMPs), conserved microbial motifs that are
recognized by pattern recognition receptors (PRRs) of the host that are able to induce innate immune responses. We characterized immune
and central responses of various MAMPs including the bacterial peptidoglycan fragments
heptanoyl-gamma-D-glutamyl-(L)-meso-diaminopimelyl-(D)-alanine ( | ||
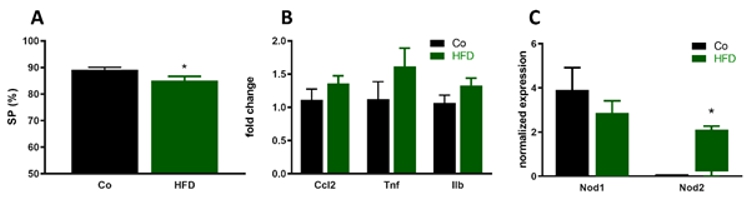
Proposed Dissertation Topic:Central effects of bacterial peptidoglycans on high fat diet-induced insulin resistance and behavioral abnormalitiesBackground: Obesity is a risk factor for cognitive impairment and mood disorders such as major depression. Western-style dietary patterns contribute to obesity-related complications through changes in gut microbiome composition, resulting in inflammatory and endocrine alterations that ultimately affect brain function and behavior. Specifically, challenging mice with a high-fat diet (HFD) leads to central inflammatory processes and insulin resistance that both contribute to synapto-dendritic and behavioral abnormalities. Interestingly, bacterial cell-wall-derived muramyl dipeptide (MDP) blunted HFD-induced adipose tissue inflammation and glucose intolerance by signaling through Nod2 and the transcription factor interferon regulatory factor 4 (IRF4), whereas Nod1 activation exerted opposite effects. However, the central effects of these peptidoglycan derivates in the context of HFD-induced obesity are unknown. Potential central effects of peptidoglycans in the context of obesity are, however, of great importance, as they cross the blood brain barrier and affect central immune responses. Hypothesis and objectives: We hypothesize that Nod1 and Nod2 signaling plays an important role in HFD-induced cognitive impairment and depression by modulating immune activation and insulin resistance in the brain. Our aims are to delineate the effects of bacterial peptidoglycans, including the Nod1 agonist FK565 and the Nod2 agonist MDP, on HFD-induced changes in central immune parameters, central insulin resistance, brain function, and behavior. Furthermore, we aim to assess differences in downstream signaling in response to Nod1 and Nod2 activation.
Methods and approaches: The PhD candidate will perform in vivo mouse studies that involve the analysis of Nod1 and Nod2
signaling on HFD-induced behavioral disturbances and associated changes in brain function. To this end, test batteries of affective
behavior will be used to study social interaction, depression-like behavior, anxiety, learning, and memory (1st and 2nd year). In addition,
the student will perform insulin sensitivity and glucose tolerance tests and determine central insulin signaling by quantification
of insulin receptor expression and insulin receptor substrate 1 phosphorylation in various brain areas. Downstream signaling in response
to Nod1 and Nod2 activation will be assessed by IRF4 expression and NFκB activity assessed by TransAM® NFκB Activation
Assay. Central immune activation will be analyzed by quantifying pro-inflammatory cytokines (TNFα, Pitfalls and alternative approaches: The methods and techniques used in this project are established and part of routine lab work, so we do not expect to encounter major technical problems. Previously, we administered the Nod agonists via intraperitoneal administration. However, recent research has demonstrated that peptidoglycan trafficking is activated by the gut microbiota, and oral administration surprisingly leads to higher central levels of peptidoglycans [15]. Consequently, we will administer the Nod agonists under study via the oral route. Importantly, exogenously administered peptidoglycan results in high concentrations of peptidoglycan in brain tissue, highlighting the significance of central peptidoglycan signaling. If, contrary to expectation, we fail to observe central effects of the Nod agonists under study, we will expand our analysis to other peptidoglycan-responsive pattern-recognition receptors known to have central effects, such as PGN-recognition protein 2 [16]. Involved Faculty members: Aitak Farzi (PI), Julia Kargl (flow cytometry), Dagmar Kratky (metabolic phenotyping), Stefano Angiari (Nod signaling). International Collaborations: Kenny (Chi Kin) Ip (Garvan Institute of Medical Research, Sydney, AUS). Facilities: Our team currently consists of one PhD candidate, one master student, personnel trained in mouse maintenance and handling, and one technicians. Our laboratory is located on the newly opened basic research campus of the university, just opposite the university hospital. Our division provides the required laboratory and office space, secretarial assistance, and basic laboratory facilities, such as laboratories for behavioral experiments, flow cytometry, immunofluorescence microscopy and more. Specific equipment for mouse behavioral testing are also available in our lab. LabMaster Metabolic Cage system for metabolic phenotyping, electron microscopy and metabolomics are also available on the campus through collaboration partners. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)