|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Simon SEDEJ
|
| Acad. Degree:
| Associate Professor, PhD
|
| Current Position:
| Research Group Leader “Basic and Translational Research on Cardiac Aging and Heart Failure”,
Division of Cardiology and University Heart Center,
Medical University of Graz
|
| Contact Details:
|
Division of Cardiology, Medical University of Graz,
Auenbruggerplatz 15, A-8036 Graz;
phone: +43 316 385 74742,
✉ e-mail
|
| Websites:
|
[Personal]
|
| ORCID:
|
[0000-0002-4419-6821]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
Our research focuses on the fundamental mechanisms of cardiac aging and heart insufficiency with the aim to identify and validate molecular
candidates as effective therapeutic interventions against cardiovascular aging, cardiometabolic syndrome, and heart failure with preserved
ejection fraction (HFpEF). To tackle this most common chronic cardiovascular disease, we combine whole body physiological and
cell- / tissue-specific molecular approaches using aged mouse cohorts, appropriate transgenic animal models, and human liquid
and myocardial biopsies from failing and non-failing donors.
We pioneered the application of caloric restriction mimetics in cardiovascular medicine, demonstrating cardioprotection by spermidine
against aging and hypertension [1]. We found that dietary supplementation of spermidine to rodents improves cardiac
autophagy and mitophagy, the relative size and function of mitochondria and myofilaments, and mechano-elastic properties of cardiomyocytes
through enhanced titin phosphorylation. Furthermore, we found that spermidine improves the systemic inflammatory status associated not only
with aging but also hypertension-induced heart failure. These results suggest dietary intake of spermidine as a novel and feasible strategy
against cardiovascular disease and provide new insights into the healthy cardiac aging, with direct clinical implications. This
translational work paved the way to a clinical study, investigating anti-hypertensive potential of spermidine-rich therapy in patients that
are non-responsive to standard medication (Clinical Trials Identifier: NCT04405388).
In 2021, we contributed to the international fight against HFpEF by demonstrating that boosting NAD+ metabolism by nicotinamide
or other precursors might become a therapy for HFpEF [2]. We showed that (i) cardiac NAD+ levels are
significantly reduced in HFpEF patients and in rats with diastolic dysfunction; (ii) oral supplementation of the NAD+
precursor nicotinamide exerts cardiometabolic benefits, thereby improving cardinal signs of HFpEF in three different animal models of
diastolic dysfunction, induced by metabolic syndrome, hypertension or advanced age; (iii) nicotinamide reduces immune cell
infiltration in the adipose tissue of rats with HFpEF. In a large prospective human cohort with 20 years of follow-up, we demonstrated
that dietary intake of naturally-occurring NAD+ precursors is associated with lower blood pressure and a reduced risk of cardiac
mortality.
Our latest work provides compelling evidence that the relationship between cardiac insulin / insulin-like growth
factor-1 (IGF-1) receptor signaling and healthspan is not linear as currently viewed, but rather biphasic and
age-dependent [3]. Mechanistically, we uncovered that reduced cardiac autophagic flux and mitochondrial oxidative
capacity underlay the late-life detrimental effects of IGF-1 receptor signaling. This study reveals how regulation of the
cardiac IGF-1 receptor signaling in the course of aging promotes health and longevity. Late-life and cell-specific targeting
of the IGF-1 signaling pathway might be the solution to harness the geroprotective potential of IGF-1 signaling,
and is expected to ignite a paradigm shift in the ongoing pursuit to delay human aging.
|
Proposed Dissertation Topic:
Immunometabolic crosstalk in heart failure with preserved ejection fraction
Background:
Metabolic alterations and immune dysregulation are associated with impaired autophagy, which collectively play critical roles in the
pathogenesis of heart failure with preserved ejection fraction (HFpEF) [4]. While systemic inflammation is clearly
implicated in this obesity-related syndrome, little is known about the specific immune cells that might trigger such metabolic inflammation.
However, a population of cardiac-resident macrophages with a pro-inflammatory phenotype promotes myocardial fibrosis and stiffness in
non-obese models of diastolic dysfunction, which is a hallmark of HFpEF [5]. By contrast, macrophages in the healthy
heart prevent metabolic dysfunction and inflammasome activation by scavenging and eliminating cardiomyocyte-derived waste in a process of
phagocytosis (i. e. heterophagy) [6]. Yet, it remains unclear how cardiac-resident macrophages
affect metabolically stressed cardiomyocytes in experimental HFpEF.
Hypothesis and objectives:
We speculate that macrophage autophagy maintains the homeostasis of neighboring cardiomyocytes in experimental HFpEF and, hence, might be
protective in HFpEF. The PhD candidate will (i) determine whether autophagy-deficient monocytes / macrophages exacerbate
HFpEF; (ii) identify consequences of monocyte / macrophage autophagy inhibition in HFpEF; (iii) elucidate whether
macrophage autophagy is required for HFpEF therapy by NAD+, which is known to affect both inflammation and autophagy
[2, 7].
Methods and approaches:
The student will be trained to perform transthoracic echocardiography to assess the impact of impaired autophagy in macrophages on
diastolic dysfunction (1st year); metabolic phenotyping to elucidate changes in insulin sensitivity, glucose homeostasis, and
plasma / tissue metabolome to explore alterations of systemic vs. tissue-specific metabolism (heart and white adipose tissue)
upon macrophage autophagy inhibition. Histological analysis will be used to detect and quantify inflammatory lesions and fibrosis. The
quantity and phenotype of infiltrating myeloid cell populations and tissue-resident macrophages will be assessed by FACS-based
immunophenotyping. This will be complemented with the profiling of local and circulating cytokines and chemokines (1st and 2nd year);
high-resolution imaging to assess cardiac autophagic flux and mitophagy, cardiomyocyte oxidative stress, apoptosis, and mitochondrial
biology (3rd year). Finally, the student will test an NAD+ precursor in the mouse model with impaired autophagy in
monocyte / macrophages (3rd and 4th year).
Pitfalls and alternative approaches:
- Because female sex provides protection against HFpEF in C57BL/6N mice, we will use mice on a C57BL/6J background. This strain,
like humans, exhibits higher susceptibility to HFpEF in females [8]. In fact, as opposed to typically used
male mice, human HFpEF disproportionately affects women [9]. Furthermore, young C57BL/6J female mice fed HFD
and L-NAME are more vulnerable to diastolic dysfunction and HFpEF than young males [8]. Since
we anticipate sex-specific differences in HFpEF phenotype, we will include the same number of male and female mice in each
experimental group.
- In case that cardiac-resident macrophages will affect metabolically stressed cardiomyocytes in a mouse model of HFpEF, we will
perform co-culture studies to decipher the mechanisms underlying the crosstalk between cardiac macrophages and cardiomyocytes.
- In an unlikely scenario that NAD+ replenishment by nicotinamide will fail to exhibit the anti-inflammatory effects and therapeutic
efficacy, we will use another NAD+ booster, e. g., nicotinamide mononuleotide or nicotinamide riboside.
Involved Faculty members:
Simon Sedej (PI), Julia Kargl (FACS-based immune profiling), Susanne Sattler
(cardiac immune cell infiltration), Dagmar Kratky (endothelial and vascular dysfunction).
International Collaborations:
Guido Kroemer (Paris, France) and María Mittelbrunn (Madrid, Spain).
Facilities:
Our team currently consists of three PhD candidates and a technician. Our research group is based in the
Center for Medical Research, and has strong expertise in applying live-cell
imaging techniques that provide spatial and temporal information of dynamic events in single cells, and in vivo cardiac and
metabolic profiling methods, which include a wide spectrum of cutting-edge techniques:
- setup for invasive hemodynamics (pressure-volume measurements)
- transthoracic echocardiography
- non-invasive blood pressure measurements
- indirect calorimetry coupled to treadmill
- indirect calorimetric and metabolic phenotyping in home cages
Our laboratory is equipped with a laser scanning confocal microscopy setup, and an epifluorescent microscope combined with a cardiomyocyte
contractility recording system. The wide spectrum of state-of-the-art techniques and know-how is complemented by histological analysis and
standard protein biochemistry and molecular biology methods. All of the necessary infrastructure and sufficient lab and office space is
available to conduct the planned experimental work. The exceptional spectrum of laboratories, equipment and core facilities, including
state-of-the-art animal facilities (for housing and breeding) with animal care personnel as well as specialist veterinary support is
concentrated within the campus of the Medical University of Graz, which offers an excellent and stimulating scientific environment.
Preparatory Findings:
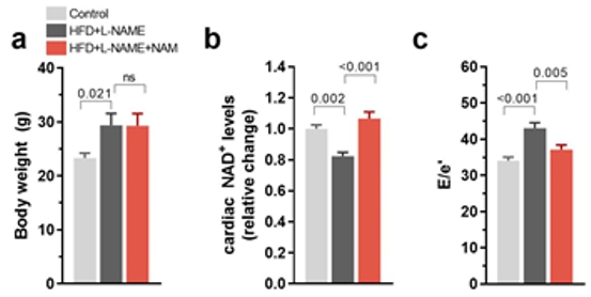
Figure 1. Nicotinamide improves cardiometabolic syndrome-induced diastolic dysfunction in young mice.
(a) Body weight, (b) cardiac NAD+ levels, and (c) diastolic dysfunction in
20-week-old C57BL/6J male mice fed 60 % high-fat diet and L-NAME in the absence or presence of 40 mM
nicotinamide (NAM, 0.5 % w / v in the drinking water) for 10 weeks;
n = 9–10 mice per group. Bars and error bars show mean and SEM. Abbreviations: E/e′, diastolic
dysfunction measured by echocardiography-derived ratio of peak early Doppler transmitral flow velocity (E) to myocardial tissue Doppler velocity
(e'). ANOVA with Dunnett’s post-hoc test.
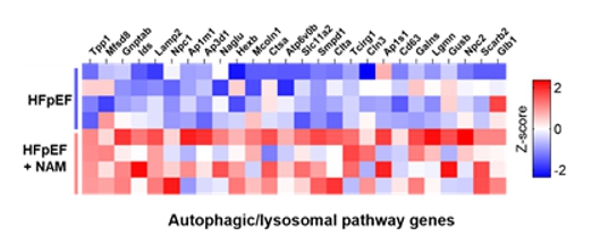
Figure 2. Oral nicotinamide supplementation increases cardiac expression of regulated genes involved in the autophagic-lysosomal pathway.
Heatmap (red = high, blue = low). Total RNA was isolated from cardiac tissue of HFpEF animals treated or not with
nicotinamide; n = 4 animals per group.
|
|
References:
-
Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M,
Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K,
Carmona-Gutierrez D, Büttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M,
Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G,
Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA,
Mühlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F:
Cardioprotection and lifespan extension by the natural polyamine spermidine.
Nat Med,
2016; 22(12):1428–1438.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, Freundt JK, Voglhuber J, Pricolo MR,
Kasa M, Türk C, Aprahamian F, Herrero-Galán E, Hofer SJ, Pendl T, Rech L, Kargl J, Anto-Michel N, Ljubojevic-Holzer S,
Schipke J, Brandenberger C, Auer M, Schreiber R, Koyani CN, Heinemann A, Zirlik A, Schmidt A, von Lewinski D, Scherr D,
Rainer PP, von Maltzahn J, Mühlfeld C, Krüger M, Frank S, Madeo F, Eisenberg T, Prokesch A, Leite-Moreira AF,
Lourenço AP, Alegre-Cebollada J, Kiechl S, Linke WA, Kroemer G, Sedej S:
Nicotinamide for the treatment of heart failure with preserved ejection fraction.
Sci Transl Med,
2021; 13(580):eabd7064.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Abdellatif M, Trummer-Herbst V, Heberle AM, Humnig A, Pendl T, Durand S, Cerrato G, Hofer SJ, Islam M, Voglhuber J, Ramos Pittol JM,
Kepp O, Hoefler G, Schmidt A, Rainer PP, Scherr D, vonLewinski D, Bisping E, McMullen JR, Diwan A, Eisenberg T, Madeo F,
Thedieck K, Kroemer G, Sedej S:
Fine-Tuning Cardiac Insulin-Like Growth Factor 1 Receptor Signaling to Promote Health and Longevity.
Circulation,
2022; 145(25):1853–1866.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Sedej S, Abdellatif M:
Metabolic therapy for managing heart failure with preserved ejection fraction.
Mol Cell Cardiol,
2022; 168:68–69.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT,
Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F,
Nahrendorf M:
Cardiac macrophages promote diastolic dysfunction.
J Exp Med,
2018; 215(2):423–440.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Nicolás-Ávila JA, Lechuga-Vieco AV, Esteban-Martínez L, Sánchez-Díaz M, Díaz-García E,
Santiago DJ, Rubio-Ponce A, Li JL, Balachander A, Quintana JA, Martínez-de-Mena R, Castejón-Vega B, Pun-García A,
Través PG, Bonzón-Kulichenko E, García-Marqués F, Cussó L, A-González N, González-Guerra A,
Roche-Molina M, Martin-Salamanca S, Crainiciuc G, Guzmán G, Larrazabal J, Herrero-Galán E, Alegre-Cebollada J, Lemke G,
Rothlin CV, Jimenez-Borreguero LJ, Reyes G, Castrillo A, Desco M, Muñoz-Cánoves P, Ibáñez B, Torres M, Ng LG,
Priori SG, Bueno H, Vázquez J, Cordero MD, Bernal JA, Enríquez JA, Hidalgo A:
A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart.
Cell,
2020; 183(1):94–109.e23.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Abdellatif M, Sedej S, Kroemer G:
NAD+ Metabolism in Cardiac Health, Aging, and Disease.
Circulation,
2021; 144(22):1795–1817.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Cao Y, Vergnes L, Wang YC, Pan C, Chella Krishnan K, Moore TM, Rosa-Garrido M, Kimball TH, Zhou Z, Charugundla S, Rau CD, Seldin MM, Wang J,
Wang Y, Vondriska TM, Reue K, Lusis AJ:
Sex differences in heart mitochondria regulate diastolic dysfunction.
Nat Commun,
2022; 13(1):3850.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Dewan P, Rørth R, Raparelli V, Campbell RT, Shen L, Jhund PS, Petrie MC, Anand IS, Carson PE, Desai AS, Granger CB, Køber L,
Komajda M, McKelvie RS, O'Meara E, Pfeffer MA, Pitt B, Solomon SD, Swedberg K, Zile MR, McMurray JJV:
Sex-Related Differences in Heart Failure With Preserved Ejection Fraction.
Circ Heart Fail,
2019; 12(12):e006539.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
|
|






![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)