


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Ákos HEINEMANN | |
| Univ.-Prof. Dr. med. univ. (MD) | ||
| Director Otto Loewi Research Center,
Division Head of Pharmacology, Medical University of Graz | ||
|
Otto Loewi Research Centre, Division of Pharmacology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Otto Loewi Research Centre] [Pharmacology] [Team] [Personal] | ||
| [0000-0002-8554-2372] | ||
| [semanticscholar] | ||
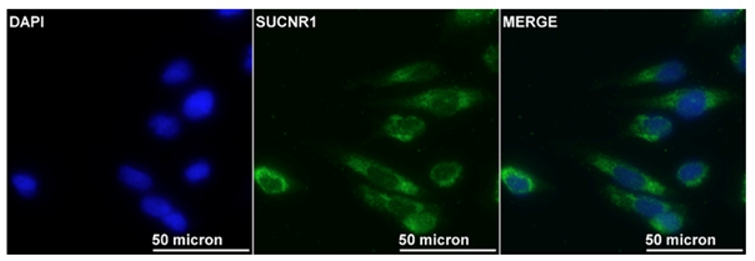
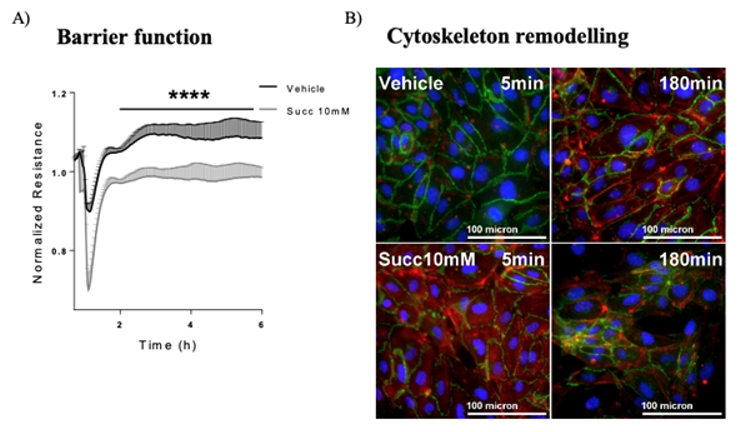
Scientific Interests:The endothelium is the gatekeeper of leukocyte trafficking into tissues and, hence, of inflammation. In the past two decades my group has characterized several small molecules and their receptors in the regulation of leukocyte trafficking and endothelial barrier function, and demonstrated their potential therapeutic potential in mouse models of pulmonary diseases and cell models of patients’ samples. Prostaglandin (PG) D2 is released from activated mast cells in allergic responses [1], but also from monocytes / macrophages in non-allergic inflammation [2, 3]. We described several novel small molecule antagonists of CRTH2, one of the receptors for PGD2, in allergic diseases and non-allergic inflammation and their efficacy to inhibit eosinophil [4, 5, 6], innate lymphoid cell (ILC2)[7] and neutrophil responses [8].Another prostaglandin, PGE2, has also been in the focus of research. We could delineate its protective and anti-inflammatory effects in the lung as a bundle of mechanisms in different cell types mediated by the G protein-coupled receptor, EP4 [9]. Activation of EP4 receptors attenuates eosinophil recruitment and activation [10], ILC2 activity [11], and cytokine secretion [12], but strengthens the endothelial barrier [12, 13]. Deposition of collagen, proliferation of myofibroblasts and recruitment of fibrocytes is likewise reversed by blockade of PGE2 metabolism [14]. We observed corresponding beneficial effects of EP4 activation in mouse models of allergic airway inflammation, acute lung injury and lung fibrosis [10, 12, 14]. Short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, are produced in high amounts in the gastrointestinal tract by commensal bacteria and can be absorbed into the bloodstream. We observed that SCFAs were able to attenuate human eosinophils at several functional levels, including (i) adhesion to the endothelium, (ii) migration, and (iii) survival. These effects were independent from GPR41 and GPR43 but were mediated by histone deacetylase inhibition. In vivo treatment with butyrate ameliorated allergen-induced airway eosinophilia, reduced type-2 cytokine levels in bronchial fluid, and improved airway hyperresponsiveness in mice [15]. Further, butyrate enhanced barrier function of endothelial cells. In contrast, the classical intermediate Krebs cycle metabolite, succinate [16], reduced endothelial barrier function in HUVECs and prompted hallmarks of angiogenesis such as proliferation, migration and spheroid sprouting in fetoplacental arterial endothelial cells (FpECAs), where it also induced endothelial tube formation. We also measured significantly higher succinate levels in placental tissue lysates from women with gestational diabetes mellitus (GDM) and found that the succinate receptor SUCNR1 was upregulated in GDM tissue lysates as well as in isolated diabetic fetoplacental arterial FpECAs. A positive correlation of SUCNR1 and vascular endothelial growth factor (VEGF) protein levels in tissue lysates indicated a potential link between the succinate-SUCNR1 axis and placental angiogenesis [17]. | ||
Proposed Dissertation Topic:Succinate-SUCNR1 regulates interaction between immune and endothelial cells in acute inflammationBackground: In the course of inflammation, metabolic rewiring of immune cells alters the levels of diverse metabolites. This is particularly the case in acute inflammatory responses, as observed in sepsis and acute lung injury. Succinate, a classically known intermediate metabolite in Krebs cycle, accumulates in macrophages stimulated with lipopolysaccharide (LPS) [18]. We have recently demonstrated that succinate triggers angiogenesis in human umbilical vein endothelial cells via its cognate receptor GPR91 / SUCNR1. However, whether other endothelial responses are also regulated via the succinate – SUCNR1 axis is not yet clear, and the role of succinate in lung disease is completely unknown. A recent study has shown that lactate from bone-marrow-derived inflammatory neutrophils facilitates their mobilization through a GPR81-dependent disruption of the endothelial barrier [19].
Hypothesis and objectives: We hypothesize that the succinate – SUCNR1 axis regulates important
endothelial parameters, such as barrier function and adhesion properties and therefore might play a crucial role in the crosstalk between
circulating immune cells and endothelial cells in acute inflammatory conditions such as acute lung injury. In detail, the student will
investigate whether (i) circulating human immune cells (
Methods and approaches: The recruited PhD candidate will be trained in and perform isolation of different immune cell
populations from donors’ blood, in vitro culture of immune cells and endothelial cells, mass-spectrometry-based
metabolomics to quantify succinate and related metabolites in the cells and in their conditioned media (1st year);
impedance measurements to investigate the integrity of endothelial barrier, video microscopy to study the adhesion of leukocytes to
endothelial cells, multi-color flow cytometry and ELISAs to quantify the expression of adhesion molecules, Western blot, qPCR and siRNA
techniques to quantify and target SUCNR1 in endothelial cells (1st and 2nd year); live-cell calcium imaging, phosphoproteomics, and cAMP
measurement amongst others to investigate the signaling machinery downstream of SUCNR1 in endothelial cells and to identify candidate
molecules to inhibit these responses (3rd year). Finally, the student will use suitable mouse models (
Pitfalls and alternative approaches:
From a technical stand, isolation of immune cells from donors’ blood is well established in the applicant’s laboratory and ethical approval
for this procedure is in place. Culture and handling of human endothelial cells from different vascular beds is also well established. All
methods and techniques planned for this project are performed regularly either in the applicant’s laboratory or by collaborators within the
frame of the university or beyond. While planning the proposed project, avoidance of artificial overexpression cellular systems is intended.
Using primary human cells with the receptor at its native expression levels will be the closest we can get to the in vivo biosystem.
However, we are aware that specific GPCRs can promiscuously couple to and activate multiple G proteins and noting the low expression levels
of GPCRs, the study of their downstream signaling at endogenous levels might be challenging. If this is the case, we will transfect the cells
to stably express SUCNR1. Furthermore, the possibility that SUCNR1 activation only potentiates the effects of other factors regulating endothelial
adhesion without exerting effects by its own cannot be excluded, Therefore, we might need to use succinate in combination with other established
substrates / ligands to induce cellular response such as Involved Faculty members: Ákos Heinemann (PI), Eva Sturm (mouse models and lung function testing), Susanne Sattler (myocardial infarction model), Dagmar Kratky (metabolic phenotyping), Stefano Angiari (SUCNR1 signaling). International Collaborations: Marc Rothenberg (Cincinnati, OH), Evi Kostenis (Uni Bonn, DE), Thomas Vorup-Jensen (Uni Aarhus, DK), James Pease (Imperial College, London, UK), Jenny Mjösberg (Karolinska, SE). Facilities: Our team currently consists of two PhD candidates, two postdocs and two technicians. Our laboratory is located on the newly opened basic research campus of the university, just opposite the university hospital. Our division provides the required laboratory and office space, secretarial assistance, and basic laboratory facilities, such as cell culture, flow cytometry, immunofluorescence microscopy and more. Specific equipment for mouse lung function testing and endothelial functional assays (interaction of endothelial cells and immune cells; endothelial barrier function) are also available in our lab. We are constantly isolating fresh human peripheral blood leukocytes from blood donors. Seahorse technology, metabolomics and mass spectrometry are also available on the campus through collaboration partners. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)