


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Martin Helmut STRADNER | |
| Assoc. Prof. Dr. med. univ. (MD) | ||
| Head of Diagnostic Immunology,
Deputy Head of the Division of Rheumatology and Immunology | ||
|
Division of Rheumatology and Immunology, Medical University of Graz,
Auenbruggerplatz 15, A-8036 Graz;
| ||
| [Team] [Personal] | ||
| [0000-0002-7884-6626] | ||
| [semanticscholar] | ||
Scientific Interests:Immunosenescence in Autoimmunity
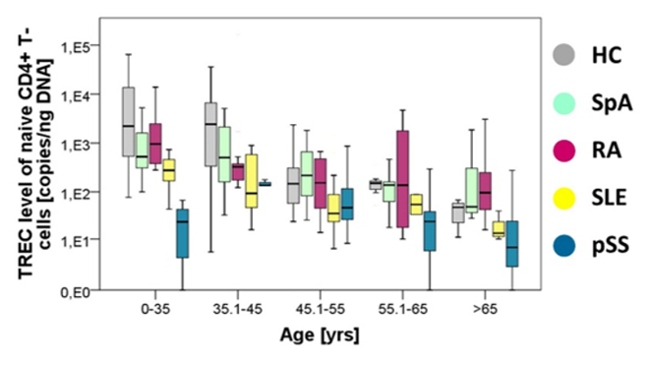
Signs of immune cell aging occur early in life in patients suffering from autoimmune diseases. Even naïïve T cells, which have
not encountered antigen yet show signs of premature aging in young patients (Fig. 1). Senescent naïve
T cells are characterized by telomerase insufficiency, increase of senescence-associated | ||
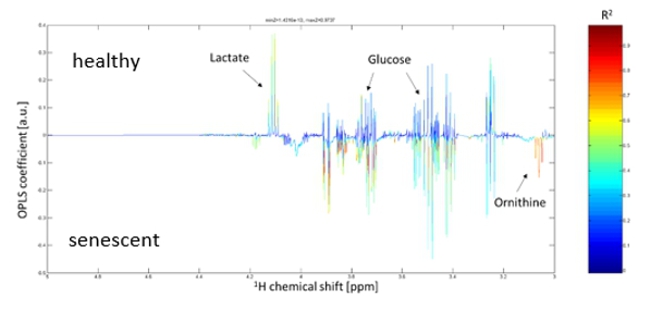
Proposed Dissertation Topic:Metabolic alterations of naïve T cells in the pathogenesis of systemic lupus erythematosusBackground: Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease associated with considerable morbidity and mortality. T cells play a critical role in the pathogenesis of SLE. We have previously shown that naïve CD4+ T cells of young SLE patients show features of premature senescence and extensive post-thymic cell division [1]. These hallmarks of immunosenescence have been closely linked to metabolic alterations including mitochondrial dysfunction, increase in reactive oxygen species (ROS), defective autophagy, and deregulated nutrient sensing [4]. Intriguingly, mitochondrial hyperpolarization towards increased glucose-derived oxidative phosphorylation resulting in increased production of ROS and active mTOR signalling are hallmarks of SLE T cells [5]. Furthermore, our preliminary data indicate increased glycolysis in senescent naïve CD4+ T cells — a feature known to be deregulated in T effector cells in SLE [5].
Hypothesis and objectives:
We hypothesize that premature immunosenescence of naive T cells drives metabolic changes in T cells associated with SLE
pathogenesis. The PhD candidate will (i) define the metabolic characteristics of senescent naïve T cells from SLE patients;
(ii) analyse the impact of immunosenescence on T cell receptor (TCR) signalling and T effector cell polarization;
(iii) unveil the molecular and mechanistic basis of these findings by
Methods and approaches:
To achieve the objectives, the PhD candidate will analyse mitochondrial biology (mitochondrial mass, membrane potential, mitochondrial
reactive oxygen species), autophagic activity, lipid metabolism and accumulation of lipid droplets, overall cellular stress via
determination of reactive oxygen species, energetic metabolism using SCENITH and seahorse technology in naïve T cells of SLE
patients and healthy controls. These findings will be associated with analyses of cellular senescence and post-thymic cell division such
as telomere length, levels of senescence-associated
Pitfalls and alternative approaches:
In case no suitable mouse model is available to address the metabolic pathways identified, the PhD candidate will extend the in vitro
analyses in the 3rd year. Using chemical modifiers of the metabolic pathways identified the student will examine its influence on Involved Faculty members: Martin H. Stradner (PI), Simon Sedej (autophagy), Dagmar Kratky (lipid metabolism), Stefano Angiari (glucose metabolism) and Kathrin Eller (mouse models). International Collaborations: Divi Cornec (Brest, France) and Ananda Goldrath (San Diego, USA). Facilities: The Stradner lab is a team of two post docs, a lab manager, two PhD candidates and several Master students. The team has access to samples of more than two thousand patients with autoimmune diseases. It is equipped with facilities for cell culture, immunofluorescence, western blot, real-time qPCR, multiplex cytokine analysis, automated cell sorting, and two high end flow cytometers. Furthermore, we have established single cell energetic metabolism by profiling translation inhibition (SCENITH). The lab has full access to the animal facilities, Seahorse XFe96 Analyzer, Cytek Aurora (Spectral Flow Cytometry) and single cell RNAseq pipeline using the Chromium Controller (10X Genomics). NMR metabolomics is performed in a long-standing cooperation with Tobias Madl at Molecular Biology and Biochemistry of our University. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)