


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Florian REICHMANN | |
| Ass.-Prof. Priv.-Doz. Dr. med. univ. (MD), PhD | ||
| Tenure Track Assistant Professor for Behavioural Neuropharmacology at Otto Loewi Research Center,
Division of Pharmacology, Medical University of Graz | ||
|
Otto Loewi Research Centre, Division of Pharmacology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Otto Loewi Research Centre] [Pharmacology] [Team] [Personal] | ||
| [0000-0002-5833-3698] | ||
| [semanticscholar] | ||
Scientific Interests:The overall focus of my research group is Behavioral Neuropharmacology. Within this area, we are trying to uncover novel mechanisms and treatment targets mediating changes in brain function considering genetic, immunological, neuronal and glial factors. This multidisciplinary work also includes the investigation of the complex communication pathways between the gut microbiome, host cells and the brain (microbiota–gut–brain axis) that are relevant for neurobiology and pathological behavioral changes. The overall goal hereby is to find new therapeutic targets for pathological behavioral changes in the context of CNS diseases or psychiatric comorbidities in the context of chronic inflammatory gastrointestinal diseases.
One of my specific interests within the research area is to investigate the effects of experimental colitis (DSS colitis), an animal model
of ulcerative colitis (UC), on brain, behavior and neurobiology. Since it has been known for a long time that patients with UC suffer from
psychiatric diseases such as depression or anxiety disorders more frequently than comparable populations [1], our goal
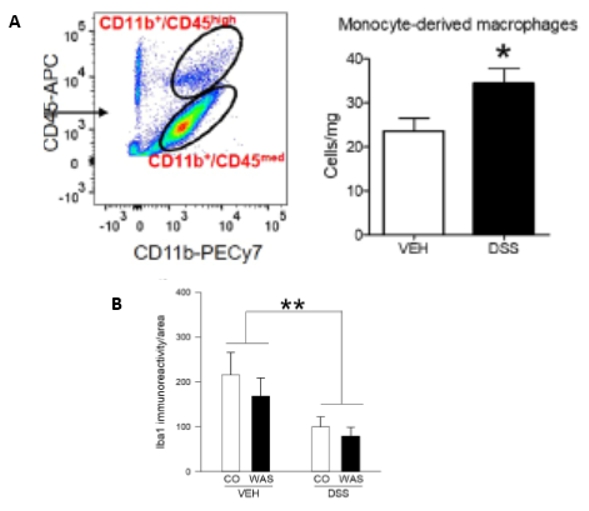
is to better understand the underlying mechanisms. For example, we have shown that mice with experimental colitis have reduced
stress-induced neuronal activation in the limbic system [2], which suggests that inflammatory signals as part of the
peripheral inflammatory process reach the brain to affect neuronal activation. In follow-up studies we have shown that animals with this
type of inflammation also show behavioral abnormalities in relation to social behavior, anxiety and pain perception
[3, 4]. In another study, we found that mice with intestinal inflammation have increased
blood levels of proinflammatory cytokines and that some of these mediators (interleukin 6 and Besides gut–brain axis signaling, we are also interested in the genetic basis of behavior. Specifically, we have examined the effects of selected gene deletions on aggressive behavior and neurobiology using the zebrafish model [7, 8]. For example, we were able to show, that genetic inactivation using CRISPR/Cas9 and pharmacological blockade of the histamine H3 receptor reduce aggressive behavior [8]. We were also able to identify new genes with aggression-reducing therapeutic potential in another study using the zebrafish model in combination with selective breeding, video tracking and RNA sequencing [9]. Current efforts focus on cross species studies using both mice and zebrafish to unravel novel conserved mechanisms of disease in the field of microbiota-gut-brain axis signaling, but also other areas. | ||
Proposed Dissertation Topic:The role of tryptophan metabolites in mediating altered gut–brain axis communication during experimental colitisBackground: Inflammatory bowel disease (IBD) is a major health concern with a continuously rising incidence and prevalence. Importantly, disease processes during IBD are not restricted to the intestinal tract, but can also affect other organs, including the brain. In fact, IBD is associated with an increased risk of primary psychiatric diseases such as depression and anxiety disorders, but the underlying mechanisms remain unclear. Clinical research and data from animal models of IBD have suggested that alterations in gastrointestinal microbes and their metabolites as well as pro-inflammatory mediators and peripheral immune cells reaching the brain might play an important role in the observed behavioral changes.
Hypothesis and objectives:
We aim to better understand the interplay between peripheral inflammation, microbial metabolites, and changes in brain function by focusing on the
tryptophan metabolites Methods and approaches: The recruited PhD candidate will use the dextran sulphate sodium (DSS)-colitis model of IBD in mice and zebrafish known to alter behavior and neurobiology. To elucidate the role of the selected tryptophan metabolites during altered gut-brain axis signaling in the course of experimental colitis, dietary interventions (tryptophan-rich diet, metabolite supplementation), pharmacological tools (aryl hydrocarbon receptor (AHR) agonists and antagonists), and genetic approaches (CRISPR/Cas9 genome editing of AHR) will be used. Transgenic zebrafish reporter lines featuring fluorescently labeled macrophages (Mpeg1.1) and neutrophils (Mpx/Mpo) will be used to assess the effects of the metabolites on immune-cell trafficking from the gut to the brain and to determine central immune cell infiltration by in vivo live imaging (1st year). The effects of the metabolites on inflammation-induced changes in microglial phenotype, neuronal activity, and central immune cell composition will be analyzed by immunohistochemistry and multi-color flow cytometry in mouse models (2nd and 3rd year). The potential of tryptophan metabolites to ameliorate colonic inflammation and neuroinflammation (ELISAs, qPCR, western blot, immunohistochemistry) and behavior (3rd and 4th year) will be tested in zebrafish and mice. These studies will provide novel insights into how peripheral gut inflammation is propagated to the brain and the potential of tryptophan metabolites to modify inflammation-induced alterations in neurobiology and behavior.
Pitfalls and alternative approaches:
Although unlikely, it is possible that the selected metabolites have only minor effects on peripheral inflammation Involved Faculty members: Florian Reichmann (PI), Ákos Heinemann (immune cell functions), Julia Kargl (phenotyping of microglia / brain immune cells), Dagmar Kratky (phenotyping of mice), Vanessa Stadlbauer-Köllner (microbiota analysis). Facilities: Our team currently consists of three PhD candidates, a technician and an animal caretaker. Our laboratory is located on the newly opened basic research campus of the university and features brand new state-of-the-art equipment and infrastructure. Besides the required laboratory spaces for standard laboratory techniques, our lab has special equipment for behavioral phenotyping of mice and zebrafish and for genome editing (CRISPR/Cas9). Metabolomics and advanced imaging infrastructure is available on campus through collaboration partners and core facilities. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)