|
|


|
The MET-FLAM Faculty
Personal information:
|

|
| Name:
| Kathrin ELLER
|
| Acad. Degree:
| Univ.-Prof. Priv.-Doz. Dr. med. univ. (MD)
|
| Current Position:
| Deputy Director of the Division of Nephrology,
Deputy Dean for Doctoral Studies,
Medical University of Graz
|
| Contact Details:
|
Division of Nephrology, Department of Internal Medicine, Medical University of Graz,
Auenbruggerplatz 27, A-8036 Graz;
phone: +43 316 385 80172,
✉ e-mail
|
| Websites:
|
[Nephrology]
[Personal]
|
| ORCID:
|
[0000-0002-6214-5008]
|
| Research Metrics:
|
[semanticscholar]
|
Scientific Interests:
Glomerulonephritis (GN) is still a major issue in modern nephrology. This broad and complex group of glomerular inflammatory diseases can
rapidly cause severe kidney damage [1]. Modern therapies like immunosuppressive agents accompanied by severe side
effects are in many cases not sufficient to stop disease progression. Consequently, GN patients after many years of treatment still
progress to end-stage kidney disease with a tremendous decline in quality of life [2]. Therefore, new treatment options
are urgently needed.
The lab of Kathrin Eller has long standing experience in GN research and is evaluating immune-mechanisms and new treatment options for the
past 20 years (beyond others [3–6]). They were the first group to discover that
regulatory T cells have the potential to be therapeutically used in experimental GN [5, 7].
In the past years, they further evaluated the potential of GLP-1R agonists, which are routinely used as anti-diabetic
medication. GLP-1R agonists (GLP-1R), such as liraglutide, are already in clinical use for patients with type-2
diabetes and obesity. and have been shown to improve surrogate renal end points in large randomized controlled trials in type-2 diabetes
(T2DM) patients [8–10]. Importantly, the renal endpoints were mainly driven by a decreased
proportion of patient developing macro-albuminuria [8–10].
In line with human reno-protective effects of GLP-1RA, various murine kidney disease models showed improved outcome when
treated with a GLP-1RA. On the one hand, we showed that GLP-1RA treatment with liraglutide improved the phenotype
of a T-cell-mediated renal disease model, namely nephrotoxic serum nephritis [11]. A research group from Aachen
provided evidence of an improved renal phenotype of db/db mice, which were upregulated for GLP-1 and its metabolites by
adenovirus-transfer adenoviral overexpression of GLP-1. The improved renal phenotype by GLP-1 was independent from
the glycaemic control and possibly mediated by decreased T-cell and macrophage infiltration [12].
GLP-1R is expressed in the kidney exclusively by pre-glomerular vascular smooth muscle cells and juxtaglomerular cells
[13–15]. There, GLP-1 mediates natriuresis and diuresis, alters renal
haemodynamics and decreases systemic blood pressure [13]. It has been speculated that anti-inflammatory capacity of
GLP-1RA might further add to the improved renal outcomes since C-reactive protein levels significantly decrease
by 25–60 % in patients with T2DM treated with GLP-1RA independent of changes in fasting glucose,
body weight and body fat [16]. GLP1R is expressed on various immune cells and exerts anti-inflammatory effects on
innate immune cells as well as T cells mice and humans [17–19].
|
Proposed Dissertation Topic:
Anti-inflammatory properties of glucagon-like peptide 1 receptor agonists in inflammatory-driven kidney disease
Background:
Glucagon-like peptide 1 (GLP-1) is a widely studied gastrointestinal incretin hormone, which stimulates insulin
production and contributes to glucose metabolism in many ways [20]. GLP-1 receptor agonists
(GLP-1RAs), such as liraglutide, are currently used to treat type-2 diabetes mellitus and obesity. Large randomized controlled trials
demonstrate that GLP-1RAs significantly improve renal function in patients with diabetic nephropathy and reduce high-sensitive
C-reactive protein significantly [9]. Data suggest that kidney protection is mediated by mechanisms beyond glycemic control.
In addition to hemodynamic effects, GLP-1RAs are also speculated to have anti-inflammatory properties [13].
The GLP-1RA liraglutide was shown to inhibit the proliferation of T cells, especially of Th1 and Th17 cells, in an
inflammatory-mediated experimental glomerulonephritis model, possibly by interfering with T-cell glucose metabolism [11].
Others have shown that GLP-1RAs alter monocyte activation as well as macrophage polarization [21].
Hypothesis and objectives:
We hypothesize that improvement of the inflammatory-mediated kidney disease model nephrotoxic serum nephritis (NTS) by GLP-1RAs
is mediated by their anti-inflammatory mechanisms. We plan to distinguish whether T cells or rather monocyte / macrophages
are responsible for the GLP-1RA protective effect. Finally, glucose metabolism will be studied in both T cells as well as
monocytes treated with GLP-1RAs. In detail, the PhD candidate will investigate (i) the effects of GLP-1RA on
T-cell function in nephrotoxic serum nephritis (NTS) by using Rag1−/− mice after CD4+ T-cells
transfer of either Glp-1r−/− or wild-type mice; (ii) the NTS phenotype in CD4+
T cell as well as monocyte / macrophage-specific Glp-1r−/− mice, both of which
will help to distinguish whether GLP-1RA signaling in T cells and / or
monocyte / macrophages is critical for T-cell proliferation in NTS; (iii) how the GLP-1RA liraglutide interacts
with glucose metabolism in T cells and monocytes using multiple methods including glycolysis assays, Seahorse analyzer, and mass
spectrometry in vitro.
Methods and approaches:
The recruited PhD candidate will be trained to isolate T cells from mice, to transfer them into Rag−/− mice,
and to induce NTS. The characterization of the NTS model includes detection of albuminuria, kidney function parameters such as blood urea
nitrogen and immune complexes by ELISA, immune cell composition in secondary lymphoid organs and the kidney by flow cytometry, assessment
of tissue damage by conventional histology, and immune cell infiltration in the kidney by immunohistochemistry. Furthermore, the PhD candidate
will determine cell composition in secondary lymphoid organs and the kidney by bulk RNAseq and single-RNAseq methodology and perform T-cell
proliferation assays (1st year). In parallel, the student will generate CD4+ T-cell- and
monocyte / macrophage-specific Glp-1r−/− mice using cre/flox mice, which will be
subjected to NTS (1st and 2nd year). In addition, the PhD candidate will be trained in in vitro techniques to stimulate and
polarize T cells and monocyte / macrophages and to analyze glucose metabolism using glycolysis assays, Seahorse analyzer,
and mass spectrometry (3rd and 4th year). Since GLP-1RAs might have profound effects also via the blood–brain axis
[7], we will also perform behaviour analysis of our NTS mice in collaboration with Aitak Farzi.
Pitfalls and alternative approaches:
It is still in discussion whether GLP-1RAs do inhibit immune cells directly since myeloid cells express only low levels of the canonical
GLP-1R. Thus, it might be the case that neither macrophage specific GLP-1R knock-out nor T-cell-specific
GLP1-R knock-out mice show aggravated phenotypes after NTS. Two alternative pathways could still be responsible for the protective
GLP-1RA effect in our NTS model. First, GLP-1RA have been shown to attenuate T-cell-mediated gut inflammation
through the gut intraepithelial lymphocyte GLP-1R [6]. Thus, via influencing the gut-kidney axis, NTS might be
improved by GLP-1RAs. This could be tested by using beta7−/− mice as published by one of our collaborators
Prof. Swirski [6]. Another mechanism how GLP-1RAs might improve NTS is by influencing the brain–immune
axis as recently described [7]. Again, we have an ongoing collaboration with Prof. Drucker, who will help us to perform
experiments to unravel this axis in our NTS model. Furthermore, we already included analysis of the behaviour of our mice with the expert help of
Aitak Farzi, who is part of our consortium.
Involved Faculty members:
Kathrin Eller (PI), Aitak Farzi (behaviour analysis), Dagmar Kratky (metabolic phenotyping),
Stefano Angiari (T-cell metabolism), Martin Stradner (T-cell characterization)
and Susanne Sattler (immune-cell phenotyping).
International Collaborations:
Antal Rot (Queen Mary University of London, UK), Katalin Susztak (University of Pennsylvania, USA), Ulf Panzer (Uni Hamburg, Germany),
Hans-Joachim Anders (LMU München, Germany).
Facilities:
Our team currently consists of two PhD candidates, two postdocs and one technician. Our laboratory is located within the university hospital.
Our division provides the required laboratory and office space, secretarial assistance, and basic laboratory facilities, such as cell
culture, flow cytometry, immunofluorescence microscopy and more. Specific equipment for mouse kidney function evaluation are also
available in our lab. Seahorse technology, metabolomics and mass spectrometry as well as bulk and single-nuclear RNAseq are also available
through collaboration partners.
Preparatory Findings:
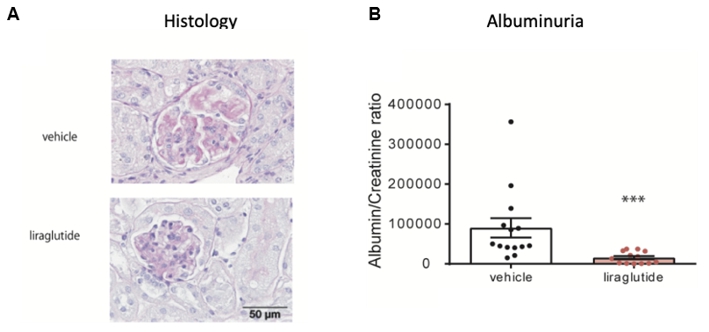
Figure 1. Liraglutide protects mice from nephrotoxic serum nephritis (NTS).
WT mice were subjected to NTS for 14 days and treated with vehicle (n = 14) or liraglutide
(n = 13) from the day of immunization. (A) Histology revealed a significantly improved
phenotype of NTS in mice treated with liraglutide. Representative PAS-stained kidney sections of mice treated with vehicle or liraglutide
are shown. Magnification ×400. (B) Urinary albumin:creatinine ratios (µg/mg) were evaluated on day 14 after
NTS induction. Liraglutide treated mice showed significantly decreased albuminuria as compared to vehicle-treated controls.
*** p < 0.001; adapted from [11].
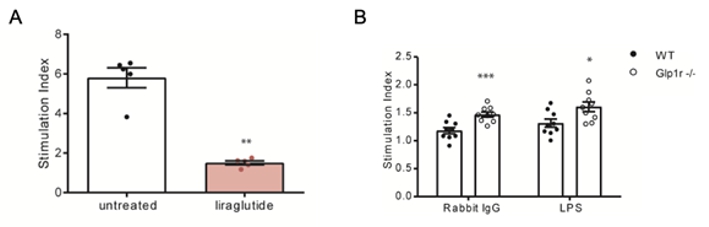
Figure 2. GLP-1R signalling decreases proliferation of T cells.
(A) T cells were isolated from C57BL/6J mice, stimulated with aCD3/CD28 and treated with liraglutide at a concentration
of 60 µg/ml (n = 5) or vehicle (n = 5). Proliferation of
cells was evaluated after 72 hours. The GLP-1RA liraglutide significantly inhibited proliferation of T cells
in vitro. (B) Splenocytes were isolated from WT (n = 9) and
Glp-1r−/− mice (n = 9) after 14 days of NTS. Cells were
stimulated in vitro by plate-coated rabbit IgG and LPS and proliferation was evaluated. Splenocytes from
Glp-1r−/− mice with NTS showed increased proliferation to both stimuli. The stimulation index
is given as the ratio between the OD values of stimulated to unstimulated cells. * p < 0.05** p < 0.01*** p < 0.001;
adapted from [11].
|
|
References:
-
Chadban SJ, Atkins RC:
Glomerulonephritis.
Lancet,
2005; 365(9473):1797–1806.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ:
KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases.
Kidney Int,
2021; 100(4S):S1–S276.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Eller K, Rosenkranz AR:
Mast Cells: Subordinates or Masterminds in Autoimmunity?
J Am Soc Nephrol,
2012; 23(12):1913–1914.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Eller K, Rosenkranz AR:
Specialized Regulatory T Cells for Optimal Suppression of T Cell Responses in GN.
J Am Soc Nephrol,
2017; 28(1):1–2.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Eller K, Weber T, Pruenster M, Wolf AM, Mayer G, Rosenkranz AR, Rot A:
CCR7 deficiency exacerbates injury in acute nephritis due to aberrant localization of regulatory T cells.
J Am Soc Nephrol,
2010; 21(1):42–52.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, Selgrade D, Fenn AM, Chan CT, Mindur JE, Valet C, Poller WC, Halle L, Rotllan N,
Iwamoto Y, Wojtkiewicz GR, Weissleder R, Libby P, Fernández-Hernando C, Drucker DJ, Nahrendorf M, Swirski FK:
Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease.
Nature,
2019; 566(7742):115–119.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Wong CK, McLean BA, Baggio LL, Koehler JA, Hammoud R, Rittig N, Yabut JM, Seeley RJ, Brown TJ, Drucker DJ:
Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation.
Cell Metab,
2024; 36(1):130&nadsh;143.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV:
Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes:
a systematic review and meta-analysis of cardiovascular outcome trials.
Lancet Diabetes Endocrinol,
2019; 7(10):776–785.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and
Investigators:
Liraglutide and Renal Outcomes in Type 2 Diabetes.
N Engl J Med,
2017; 31;377(9):839–848.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM,
Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators:
Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes.
N Engl J Med,
2016; 28;375(4):311–322.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Moschovaki Filippidou F, Kirsch AH, Thelen M, Kétszeri M, Artinger K, Aringer I, Schabhüttl C, Mooslechner AA,
Frauscher B, Pollheimer M, Niedrist T, Meinitzer A, Drucker DJ, Pieber TR, Eller P, Rosenkranz AR, Heinemann A, Eller K:
Glucagon-Like Peptide-1 Receptor Agonism Improves Nephrotoxic Serum Nephritis by Inhibiting T-Cell Proliferation.
Am J Pathol,
2020; 190(2):400–411.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Moellmann J, Klinkhammer BM, Onstein J, Stöhr R, Jankowski V, Jankowski J, Lebherz C, Tacke F, Marx N, Boor P, Lehrke M:
Glucagon-Like Peptide 1 and Its Cleavage Products Are Renoprotective in Murine Diabetic Nephropathy.
Diabetes,
2018; 67(11):2410–2419.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Muskiet MHA, Tonneijck L, Smits MM, van Baar MJB, Kramer MHH, Hoorn EJ, Joles JA, van Raalte DH:
GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes.
Nat Rev Nephrol,
2017; 13(10):605–628.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB:
GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated
monoclonal antibody.
Endocrinology,
2014; 155(4):1280–1290.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, Gribble FM, Reimann F:
Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model.
Diabetes,
2014; 63(4):1224–1233.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Bunck MC, Diamant M, Eliasson B, Corner A, Shaginian RM, Heine RJ, Taskinen MR, Yki-Jarvinen H, Smith U:
Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition.
Diabetes Care,
2010; 33(8):1734–1737.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, Makdissi A, Dandona P:
Exenatide exerts a potent antiinflammatory effect.
J Clin Endocrinol Metab,
2012; 97(1):198–207.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ:
Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of
peripheral regulatory T cells.
Diabetologia,
2010; 53(4):730–740.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Xue S, Wasserfall CH, Parker M, Brusko TM, McGrail S, McGrail K, Moore M, Campbell-Thompson M, Schatz DA, Atkinson MA, Haller MJ:
Exendin-4 therapy in NOD mice with new-onset diabetes increases regulatory T cell frequency.
Ann N Y Acad Sci,
2008; 1150:152–156.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Drucker DJ, Nauck MA:
The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in
type 2 diabetes.
Lancet,
2006; 368(9548):1696–1705.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
-
Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, Assi E, Seelam AJ, El Essawy B, Jang J, Loretelli C,
D’Addio F, Berra C, Ben Nasr M, Zuccotti G, Fiorina P:
The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists.
Pharmacol Res,
2022; 182:106320.
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)
|
|






![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)