


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Susanne SATTLER | |
| Ass.-Prof. Dr. rer. nat. (PhD) | ||
| Ass. Professor of Cardiac Immunobiology at the Division of Pharmacology, Department of Cardiology, Medical University of Graz,
Advanced Research Fellow, National Lung and Heart Institute, Imperial College London (0.2 FTE) | ||
|
Division of Pharmacologyy, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Team] [Imperial College] [Personal] | ||
| [0000-0001-9932-4109] | ||
| [semanticscholar] | ||
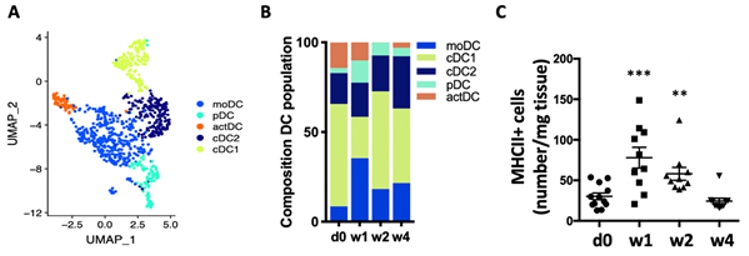
Scientific Interests:Worldwide, 64 million people are estimated to suffer from heart failure [1], a clinical condition defined as the inability of the heart to fulfil the metabolic demands of the body. Heart failure is a fatal complication of many cardiac diseases including myocardial infarction (MI), with aMany cardiomyocyte-intrinsic as well as systemic processes are under investigation to identify targetable pathways. Increasing evidence suggests that immunopathology, encompassing both local myocardial inflammation and systemic anti-heart autoimmunity entwined in a persistent inflammatory-autoimmune loop, contributes to the development of heart failure. We have previously contributed a significant body of evidence showing that not only acute innate inflammation but also the adaptive immune system is activated after MI and persistent autoimmunity contributes to the development of heart failure [4–8]. Indeed, the presence of auto-antibodies against cardiac epitopes during heart failure is a known clinical phenomenon [9], which we recently confirmed to be part of a fully established autoimmune syndrome. This includes activation and enlargement of the heart-draining lymph nodes, activated T and B cells and most importantly myocardial deposition of mature auto-antibodies and complement indicating pathological autoreactivity [6]. Autoimmunity is defined as immunological reactivity against self-antigens. It is induced under highly inflammatory conditions when peripheral tolerance mechanisms are overwhelmed. Dendritic cells (DC) are considered ‘professional’ antigen presenting cells, due to their ability to initiate T-cell activation from naïve state. DC are central in triggering autoimmunity as they determine the resulting T-cell functional phenotype depending on inflammatory context [10]. We have previously shown that they increase in numbers and upregulate their antigen-presentation capacity after MI (figure 1). In addition, the antigen-presentation machinery can be upregulated in tissue-resident cells under inflammatory conditions [11]. Importantly, aging and metabolic disease are associated with increased levels of systemic and tissue inflammation (inflammaging) [12], which has been suggested to affect antigen presentation pathways [13]. If increased antigen presentation due to metabolic disease and inflammaging has a role to play in immunopathology of the heart remains to be investigated. | ||
Proposed Dissertation Topic:How does obesity affect antigen-presentation pathways in post-ischemic cardiac immunopathology?Background: Systemic inflammation due to metabolic disease, including obesity, has been implicated in a range of cardiovascular conditions. Myocardial inflammation and an ensuing activation of adaptive immune responses and autoimmunity against cardiac antigens, is involved in the development of heart failure after a myocardial infarction (MI). Antigen-presentation by dendritic cells (DC) initiates autoimmunity, and tissue-resident mesenchymal and parenchymal cells can also contribute to antigen-presentation and may perpetuate local inflammation. The effect of obesity on antigen-presentation pathways, either through modified lipoproteins or inflammatory factors, leading to post-MI anti-heart autoimmunity and heart failure, remains to be investigated. Hypothesis and objectives: We hypothesize that obesity increases the inflammatory self-antigen-presentation in DC as well as in heart-resident cells. This contributes to an adaptive immune response against cardiac antigens exacerbating adverse remodeling towards heart failure. The PhD candidate will (i) characterize the effect of obesity on the spatio-temporal dynamics of the DC population, (ii) analyze the antigen-presentation machinery in cardiac-resident cells, (iii) confirm functional relevance of antigen-presentation in obesity-induced cardiac remodeling, and (iv) analyze the respective effect of modified lipoproteins versus inflammation in in vitro DC cultures.
Methods and approaches:
The PhD candidate will induce ischemic myocardial damage (type-2 myocardial infarction, T2MI) in high-fat diet (HFD)-fed mice (HFD-MI).
He / she will confirm cardiac functional and structural impairment by echocardiography, and perform immunophenotyping with focus
on the DC population, in the myocardium, heart-draining mediastinal lymph nodes, adipose tissue, blood and bone marrow before and after induction
of T2MI. DC will be assessed for population changes, and their functional as well as metabolic phenotype. Measures of established autoimmune
responses, including the presence of activated T cells and anti-heart auto-antibodies will be included. (1st and 2nd year). The PhD candidate
will further isolate / differentiate DC from HFD-MI mice for in vitro functional assays to assess their ability to
activate T cells against myocardial antigen. Besides DC, they will also isolate cardiac-resident cells including fibroblasts, endothelial
cells and cardiomyocytes to assess their immunophenotype by immunostaining, flow cytometry and qPCR, as well as their potential antigen-presentation
ability by in vitro co-culture assays. DC from wild-type mice will be used in vitro to assess the effect of
obesity-related modified lipoproteins
Pitfalls and alternative approaches:
Obesity is a systemic condition affecting all organ systems and a wide range of pathways, hence several results obtained throughout this study
will remain associative without showing direct causative effects. Causative effects are investigated through the included mechanistic in vitro
studies ( Involved Faculty members: Susanne Sattler (PI), Gunther Marsche (lipoprotein biology), Stefano Angiari (T-cell metabolism), Ákos Heinemann (endothelial cell assays), Michael Khalil (soluble markers of inflammation), Simon Sedej (in vivo cardiac phenotyping), Julia Kargl (immunophenotyping and DC metabolic pathways). International Collaborations: Julia Gorelik, Tristan Rodríguez, Rasheda Chowdhury (Imperial College, London, UK), Gustavo Ramos (University of Würzburg, Germany), Florian Leuschner (University of Heidelberg, Germany), Sikander Hayat (University of Aachen, Germany), Nadia Rosenthal (The Jackson Laboratories, USA), Ebba Brakenhielm (University of Rouen, France), Pilar Martín Fernández (CNIC, Spain), Kory Lavine (Washington University, USA). Facilities: Our lab is located in the Center for Medical Research (ZMF) within the campus of the Medical University of Graz. This location offers an excellent and stimulating scientific environment, formed through the local concentration of Medical University of Graz, University of Graz and Graz University of Technology. The local research group has strong expertise in the functional, structural, biochemical and molecular biological characterization of the cardiovascular system including in vivo cardiac profiling methods such as invasive haemodynamics, pre-clinical imaging equipment such as ultrahigh-resolution transthoracic echocardiography, non-invasive blood pressure measurements and set ups for metabolic experiments. Core facilities at the ZMF include state-of-the-art flow-cytometry equipment, laser-scanning confocal microscopy, an epi-fluorescent microscope combined with IonOptix Myocyte Contractility Recording System, a two-chamber oxygraph and a Seahorse assay system. Researchers at the Department of Cardiology have access to an extensive biobank of patient serum and myocardial biopsy samples obtained previously. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)