


The MET-FLAM Faculty
Personal information: |

| |
| Name: | Grażyna KWAPISZEWSKA | |
| Assoz. Prof. Priv.-Doz. Dr. (PhD) | ||
| Director Ludwig Boltzmann Institute for Lung Vascular Research, Graz
Assoc. Prof of Pulmonary Vascular Remodeling, Med Uni Graz Prof. Aberrant Remodeling & Regeneration in Chronic Lung Disease, Institute of Lung Health, Giessen | ||
|
Otto Loewi Research Centre, Division of Physiology and Pathophysiology, Medical University of Graz,
Neue Stiftingtalstraße 6, A-8010 Graz;
| ||
| [Research Centre] [Section] [Team] [Personal] | ||
| [0000-0002-8554-2372] | ||
| [semanticscholar] | ||
Scientific Interests:Our team focuses on the “vascular point of view on chronic lung diseases”. Chronic lung diseases, such as pulmonary fibrosis, are an increasing cause of death in the aging population. As vascular involvement in these diseases worsens patient outcomes, our team aims to unravel the compartment-specific role of mesenchymal cells and their interaction with immune processes and their vascular abnormalities. Based on our previous experience with lineage tracing technology, single-cell analysis and multi-color fluorescent microscopy we dissected the heterogeneity of the lung vascular and parenchymal abnormalities [1, 2, 3]. We have also shown that metabolic activation via rosiglitazone or metformin can have a beneficial outcome [4, 5].In addition, we have demonstrated the involvement of basement membrane components in the remodelling processes and recruitment of immune cells towards tissue injury [6, 7, 8]. Recently, we have focused on understanding how immune cells and their mediators influence cellular expansion and phenotypic switch of surrounding cells. Indeed, changes in immune cells composition actively effect vascular and parenchymal remodelling and thus disease progression. Immunomodulation can lead either to exacerbations of the disease or beneficial outcome [9, 10, 11, 12]. Thus, understanding the complex cellular intercommunication and metabolic alteration stay in the centre of scientific focus. | ||
Proposed Dissertation Topic:Immune cells shape the metabolic status of structural cells and lead to the lung fibrosis development in the mouse model of scleroderma
Background:
Vascular abnormalities are a common feature of chronic lung diseases such as systemic sclerosis (SSc). These vascular abnormalities can
manifest as remodeling and obliteration of pulmonary arteries, as seen in Ssc associated with pulmonary hypertension (PH). Current studies
indicate that immune cells actively modulate behavior of underlying structural cells and are involved in disease progression.
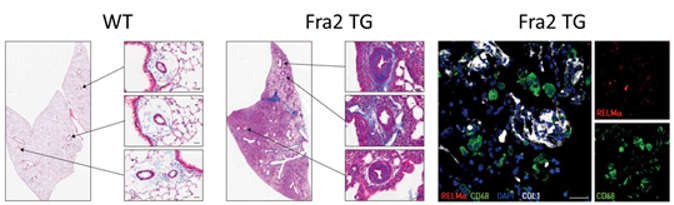
We have previously shown that transcription factor fos-related
Hypothesis and objectives:
Immune cells, by secreting a plethora of factors, can directly influence the metabolic status of underlying structural cells and thus
lead to disease development. Therefore, we hypothesize that altered cross-talk between immune and structural cells leads to vasculopathy
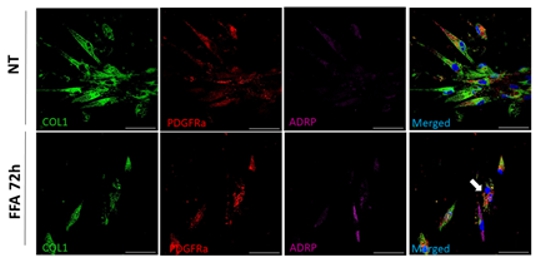
and to development of pulmonary fibrosis in Ssc Methods and approaches: In the 1st year the PhD candidate will perform isolation and characterization of different immune and structural cell populations from mouse and human lung tissue. He / she will conduct flow cytometry, cell-sorting and MACS approach, and multi-colour immunofluorescence staining to determine spatial localization of the immune cells and parenchymal cells in the healthy and fibrotic lung tissue. In the 2nd and 3rd year he / she will be dissecting the cross-talk between immune and structural cells (primary smooth muscle and fibroblast from healthy and diseased lung tissue from human and murine tissue) in co-culture systems and study the migration, proliferation and secretome of the structural cells. The receptor–ligand interaction will be analyzed in silico as well as in vitro. Spheroids and organoid models will be used to understand complex 3D interactions. Here, diverse immune cells will be injected in the spheroids / organoids and their effects on the expansion / distribution of the structural cells will be analyzed. Additionally, the metabolic status of the expanding cells will be established. In the 3rd and 4th year, the student will investigate which metabolic changes in the structural cells lead to the cellular expansion in vivo. Here, the lineage tracing mouse models [1, 2] will be applied to dissect expanding cells. Studies on human lung tissue will complement the variety of in-vitro and in-vivo approaches. Pitfalls and alternative approaches: We will employ a range of methodologies to assess the metabolic status of the cells. For instance, we will utilize NMR spectroscopy (Nuclear Magnetic Resonance) to identify characteristic metabolic patterns and elucidate associated alterations in metabolic products. Additionally, mass spectrometry (MS) will be utilized for comprehensive metabolite profiling. The energy status of the cells will be evaluated using Seahorse XF technology. These methodologies are readily available at Med Uni Graz. By employing multiple analytical techniques, we will ensure robustness in our findings, mitigating potential limitations such as sensitivity issues encountered with low cell numbers. Furthermore, our organoid studies will benefit from collaboration with Prof. Susanne Herold from Giessen, Germany, who possesses expertise in this area. In circumstances where challenges arise with human organoids, particularly concerning iPSC differentiation, we will opt for mouse organoids due to their relative ease of cultivation. Involved Faculty members: Grażyna Kwapiszewska (PI), Dagmar Kratky (metabolic phenotyping), Ákos Heinemann (immune cell isolation), Kathrin Eller (immune–structural cell cross-talk), Stefano Angiari (immunometabolism). International Collaborations: Anne-Karine Perl (Cincinnati, USA), Edward Morrissey (Pennsylvania, USA), Norbert Weissmann (Giessen, DE), Elie-El Agha (Institute for Lung Health, Giessen, DE), Karin Tran-Lundmark (Lund, SE). Facilities: Our team consists of five PhD candidates, four postdocs and two technicians. We are located on the newly opened basic research campus of the university, opposite the university hospital. Our lung group evolved from Ludwig Boltzmann Institute for Lung Vascular Research and is very closely working with the division of Pulmonology. We provide the required laboratory and office space, all necessary laboratory facilities, including cell culture, flow cytometry, multi-color immunofluorescence microscopy and animal laboratory equipment. Due to our close cooperation with German Lung Center we have also access to all cutting-edge technologies necessary for investigation of lung (patho)biology on the international level. Preparatory Findings:
| ||
|
References:
| ||
![[DOI Journal link] [DOI Journal link]](gifs/doi.gif)